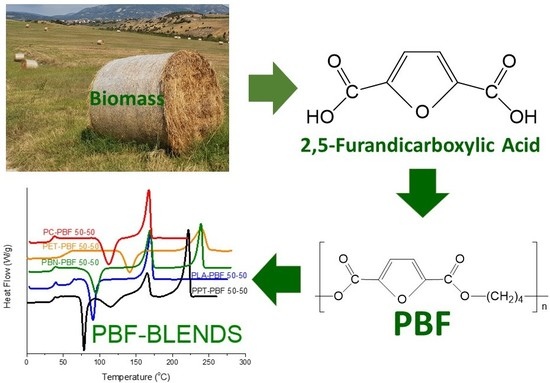
Biobased Engineering Thermoplastics: Poly(butylene 2,5-furandicarboxylate) Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Polyesters
2.2. Preparation of Polymer Blends
2.3. Characterization Methods
2.3.1. Intrinsic Viscosity Measurements
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. X-ray Diffraction
2.3.4. Polarizing Light Microscopy (PLM)
3. Results and Discussion
3.1. Synthesis of PBF
3.2. PLA–PBF Blends
3.3. PET–PBF Blends
3.4. PPT–PBF Blends
3.5. PBN–PBF Blends
3.6. PC–PBF Blends
3.7. Calculation of the Solubility Parameters of the Polymers
3.8. Polarized Light Microscopy Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 357. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-Catalyzed oxidation of 5-Hydroxymethylfurfural to Furan-2,5-dicarboxylic acid. Angew.Chem. 2014, 53, 6515–6518. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M.W. A scalable carboxylation route to furan-2,5-dicarboxylic acid. Green Chem. 2017, 19, 2966–2972. [Google Scholar] [CrossRef]
- Teles, J.H. Across the Board: J. Henrique Teles. ChemSusChem 2019, 12, 338. [Google Scholar] [CrossRef]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 Crystal Structure on Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. J. Am. Chem. Soc. 2019, 141, 890–900. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D.; Matos, M.; Freire, C.S.R. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Gandini, A. The behavior of furan derivatives in polymerization reactions. Adv. Polym. Sci. 1977, 25, 47–96. [Google Scholar]
- Moore, J.A.; Kelly, J.E. Polyesters Derived from Furan and Tetrahydrofuran Nuclei. Macromolecules 1978, 11, 568–573. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. A biocatalytic approach towards sustainable furanic–aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Ciulik, C.B.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Fully bio-based aromatic–aliphatic copolyesters: Poly(butylene furandicarboxylate-co-succinate)s obtained by ring opening polymerization. Polym. Chem. 2017, 8, 748–760. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Petrovic, D.M.; Loos, K. Enzymatic Synthesis of Biobased polyesters using 2,5-Bis(hydroxymethyl)furan as the building block. Biomacromolecules 2014, 15, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pang, Y.; Wang, M.; Xu, J.; Ma, H.; Nie, X. The copolymerization reactivity of diols with 2,5-furandicarboxylic acid for furan-based copolyester materials. J. Mater. Chem. 2012, 22, 3457–3461. [Google Scholar] [CrossRef]
- Knoop, R.J.; Vogelzang, W.; Haveren, J.; Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.-H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furan dicarboxylate), a Biobased Alternative to PBT: Synthesis, Physical Properties, and Crystal Structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Xu, J.; Pang, Y. Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Soccio, M.; Martínez-Tong, D.E.; Alegría, A.; Lotti, N. Molecular dynamics of fully biobased poly(butylene 2,5-furanoate) as revealed by broadband dielectric spectroscopy. Polymer 2017, 128, 24–30. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikiaris, D.N. Evaluation of polyesters from renewable resources as alternatives to the current fossil-based polymers. Phase transitions of poly (butylene 2,5-furan-dicarboxylate). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, J.M.; Dubois, P. High Molecular Weight Poly(butylene succinate-co-butylene furandicarboxylate) Copolyesters: From Catalyzed Polycondensation Reaction to Thermomechanical Properties. Biomacromolecules 2013, 14, 890–899. [Google Scholar] [CrossRef]
- Wu, B.; Xu, Y.; Bu, Z.; Wu, L.; Li, B.-G.; Dubois, P. Biobasedpoly(butylene 2,5-furandicarboxylate) and poly(butylene adipate-co-butylene 2,5-furandicarboxylate)s: From synthesis using highly purified 2,5-furandicarboxylic acid to thermo-mechanical properties. Polymer 2014, 55, 3648–3655. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Jacquel, N.; Saint-Loup, R.; Pascault, J.-P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic-aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Manaresi, P.; Munari, A. Novel fully biobased poly(butylene 2,5-furanoate/diglycolate) copolymers containing ether linkages: Structure-property relationships. Eur. Polym. J. 2016, 81, 397–412. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martınez de Ilarduya, A.; Munoz-Guerra, S. Blocky poly(ε-caprolactone-co-butylene 2,5-furandicarboxylate) copolyesters via enzymatic ring opening polymerization. J. Polym. Sci. A Polym. Chem. 2018, 56, 290–299. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Sustainable Aromatic Copolyesters via Ring Opening Polymerization: Poly(butylene 2,5-furandicarboxylate-co-terephthalate)s. ACS Sustain. Chem. Eng. 2016, 4, 4965–4973. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.F.; Guigo, N.; Pożycka, M.; Delgado, M.; Soares, J.; Mendonca, P.V.; Coelho, J.F.J.; Sbirrazzuoli, N.; Silvestre, A.J.D. Tailor design of renewable copolymers based on poly(1,4-butylene 2,5-furandicarboxylate) and poly(ethylene glycol) with refined thermal properties. Polym. Chem. 2018, 9, 722–731. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Xu, Y.; Wang, P.; Gao, L.; Zhang, W.; Ji, J. Synthesis and characterization of bio-based poly(butylene furandicarboxylate)-b-poly(tetramethylene glycol) copolymers. Polym. Degrad. Stab. 2014, 109, 21–26. [Google Scholar] [CrossRef]
- Cai, X.; Yang, X.; Zhang, H.; Wang, G. Aliphatic-aromatic poly(carbonate-co-ester)s containing biobased furan monomer: Synthesis and thermo-mechanical properties. Polymer 2018, 134, 63–70. [Google Scholar] [CrossRef]
- Kuang, T.; Chen, F.; Chang, L.; Zhao, Y.; Fu, D.; Gong, X.; Peng, X. Facile preparation of open-cellular porous poly (l-lactic acid) scaffold by supercritical carbon dioxide foaming for potential tissue engineering applications. Chem. Eng. J. 2017, 307, 1017–1025. [Google Scholar] [CrossRef]
- Kuang, T.; Ju, J.; Yang, Z.; Geng, L.; Peng, X. A facile approach towards fabrication of lightweight biodegradable poly (butylene succinate)/carbon fiber composite foams with high electrical conductivity and strength. Compos. Sci. Technol. 2018, 159, 171–179. [Google Scholar] [CrossRef]
- Kuang, T.; Li, K.; Chen, B.; Peng, X. Poly(propylene carbonate)-based in situ nanofibrillar biocomposites with enhanced miscibility, dynamic mechanical properties, rheological behavior and extrusion foaming ability. Compos. Part B Eng. 2017, 123, 112–123. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Zhang, J.; Rayand, N.; Hu, G.-H.; Yang, J.; Zhu, J. Retroreflection in binary bio-based PLA/PBF blends. Polymer 2017, 125, 138–143. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Jiang, Y.; Hu, G.-H.; Yang, J.; Zhu, J. Tensile Property Balanced and Gas Barrier Improved Poly(lactic acid) by Blending with Biobased Poly(butylene 2,5-furan dicarboxylate). ACS Sustain. Chem. Eng. 2017, 5, 9244–9253. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Sustainable Polymers from Renewable Resources: Polymer Blends of Furan-Based Polyesters. Macromol. Mater. Eng. 2018, 303, 1800153. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Siampani, M.; Terzopoulou, Z.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Exploring Next-Generation Engineering Bioplastics: Poly(alkylene furanoate)/Poly(alkylene terephthalate) (PAF/PAT) Blends. Polymers 2019, 11, 556. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of Poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources. Thermal behavior comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furandicarboxylate). Comparison of thermal behavior and solid statestructure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Safapour, S.; Seyed-Esfahani, M.; Auriemma, F.; Ruiz de Ballesteros, O.; Vollaro, P.; Di Girolamo, R.; De Rosa, C.; Khosroshahi, A. Reactive blending as a tool for obtaining poly(ethylene terephthalate)-based engineering materials with tailored properties. Polymer 2010, 51, 4340. [Google Scholar] [CrossRef]
- Sonchaeng, U.; Iñiguez-Franco, F.; Auras, R.; Selke, S.; Rubino, M.; Lim, L.-T. Poly(lactic acid) mass transfer properties. Prog. Polym. Sci. 2018, 86, 85–121. [Google Scholar] [CrossRef]
- Van Berkel, J.-G.; Guigo, N.; Visser, H.A.; Sbirrazzuoli, N. Chain Structure and Molecular Weight Dependent Mechanics of Poly(ethylene 2,5-furandicarboxylate) Compared to Poly(ethylene terephthalate). Macromolecules 2018, 51, 8539–8549. [Google Scholar] [CrossRef]
- Achilias, D.S.; Papageorgiou, G.Z.; Karayannidis, G.P. Isothermal and Nonisothermal Crystallization Kinetics of Poly(propylene terephthalate). J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3775–3796. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Achilias, D.S.; Karayannidis, G.P.; Bikiaris, D.N.; Roupakias, C.; Litsardakis, G. Step-scan TMDSC and high rate DSC study of themultiple melting behavior of poly(1,3-propylene terephthalate). Eur. Polym. J. 2006, 42, 434–445. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Karayannidis, G.P. Crystallization and melting behaviour of poly(butylene naphthalene-2,6-dicarboxylate). Polymer 2001, 42, 2637–2645. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Bikiaris, D.N.; Papageorgiou, G.Z. Synthesis and controlled crystallization of in situ prepared poly(butylene-2,6-naphthalate) nanocomposites. CrystEngComm 2018, 20, 3590–3600. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and Characterization of Bio-Based Polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexanedimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; ISBN 978-0-08-054819-7. [Google Scholar]









| Polymer | V (cm3/mol) | δ2 (MJ/m3)1/2 |
|---|---|---|
| PBF | 158.4 | 22.2 |
| PLA | 60.7 | 19.9 |
| PET | 144.2 | 22.0 |
| PPT | 159.2 | 21.5 |
| PBN | 223.5 | 20.2 |
| PC | 212.0 | 20.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulopoulou, N.; Kantoutsis, G.; Bikiaris, D.N.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Biobased Engineering Thermoplastics: Poly(butylene 2,5-furandicarboxylate) Blends. Polymers 2019, 11, 937. https://doi.org/10.3390/polym11060937
Poulopoulou N, Kantoutsis G, Bikiaris DN, Achilias DS, Kapnisti M, Papageorgiou GZ. Biobased Engineering Thermoplastics: Poly(butylene 2,5-furandicarboxylate) Blends. Polymers. 2019; 11(6):937. https://doi.org/10.3390/polym11060937
Chicago/Turabian StylePoulopoulou, Niki, George Kantoutsis, Dimitrios N. Bikiaris, Dimitris S. Achilias, Maria Kapnisti, and George Z. Papageorgiou. 2019. "Biobased Engineering Thermoplastics: Poly(butylene 2,5-furandicarboxylate) Blends" Polymers 11, no. 6: 937. https://doi.org/10.3390/polym11060937