Transparent, High Glass-Transition Temperature, Shape Memory Hybrid Polyimides Based on Polyhedral Oligomeric Silsesquioxane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PI-POSS Hybrid Films
2.3. Characterization
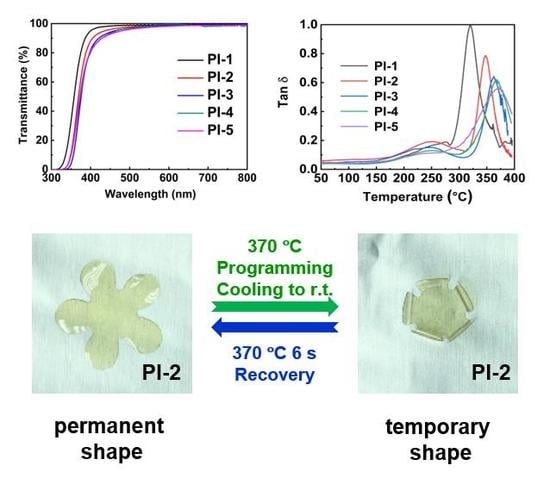
3. Results and Discussion
3.1. Thermal Behavior
3.2. Optical Properties
3.3. Shape Memory Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ji, D.Y.; Hu, W.P.; Fuchs, H. Recent Progress in Aromatic Polyimide Dielectrics for Organic Electronic Devices and Circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef] [PubMed]
- Gouzman, I.; Crossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Cicala, G.; Ognibene, G.; Rapisarda, M.; Recca, A. Thermal properties of polyetherimide/polycarbonate blends for advanced applications. Polym. Degrad. Stab. 2018, 154, 234–238. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Gao, H.; Huang, L.N.; Lan, X.; Liu, L.W.; Liu, Y.J.; Leng, J.S. The research of vacuum thermal cycling resistant transparent shape memory polyimide and its application in space flexible electronics. In Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, Portland, OR, USA, 26–28 March 2017. [Google Scholar]
- Gao, H.; Lan, X.; Liu, L.W.; Xiao, X.L.; Liu, Y.J.; Leng, J.S. Study on performances of colorless and transparent shape memory polyimide film in space thermal cycling, atomic oxygen and ultraviolet irradiation environments. Smart Mater. Struct. 2017, 26, 095001. [Google Scholar] [CrossRef]
- Xiao, X.L.; Kong, D.Y.; Qiu, X.Y.; Zhang, W.B.; Liu, Y.J.; Zhang, S.; Zhang, F.H.; Hu, Y.; Leng, J.S. Shape memory polymers with high and low temperature resistant properties. Sci. Rep. 2015, 5, 14137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.L.; Kong, D.Y.; Qiu, X.Y.; Zhang, W.B.; Zhang, F.H.; Liu, L.W.; Liu, Y.J.; Zhang, S.; Hu, Y.; Leng, J.S. Shape-Memory Polymers with Adjustable High Glass Transition Temperatures. Macromolecules 2015, 48, 3582–3589. [Google Scholar] [CrossRef]
- Xiao, X.L.; Qiu, X.Y.; Kong, D.Y.; Zhang, W.B.; Liu, Y.J.; Leng, J.S. Optically transparent high temperature shape memory polymers. Soft Matter 2016, 12, 2894–2900. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.R.; Xie, F.; Liu, Y.J.; Leng, J.S. A novel low colored and transparent shape memory copolyimide and its durability in space thermal cycling environments. Polymer 2018, 156, 121–127. [Google Scholar] [CrossRef]
- Kong, D.Y.; Xiao, X.L. High Cycle-life Shape Memory Polymer at High Temperature. Sci. Rep. 2016, 6, 33610. [Google Scholar] [CrossRef]
- Koerner, H.; Strong, R.J.; Smith, M.L.; Wang, D.H.; Tan, L.S.; Lee, K.M.; White, T.J.; Vaia, R.A. Polymer design for high temperature shape memory: Low crosslink density polyimides. Polymer 2013, 54, 391–402. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wang, Q.H.; Wang, T.M. Tunable Triple-Shape Memory Binary Mixtures with High Transition Temperature and Robust Mechanical Properties. Macromol. Chem. Phys. 2016, 217, 1305–1313. [Google Scholar] [CrossRef]
- Yang, Z.H.; Chen, Y.; Wang, Q.H.; Wang, T.M. High performance multiple-shape memory behaviors of Poly(benzoxazole-co-imide)s. Polymer 2016, 88, 19–28. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wang, Q.H.; Bai, Y.K.; Wang, T.M. AO-resistant shape memory polyimide/silica composites with excellent thermal stability and mechanical properties. RSC Adv. 2015, 5, 72971–72980. [Google Scholar] [CrossRef]
- Bai, Y.K.; Mao, L.; Liu, Y.J. High temperature shape memory polyimide ionomer. J. Appl. Polym. Sci. 2016, 133, 43630. [Google Scholar] [CrossRef]
- Wang, Q.H.; Bai, Y.K.; Chen, Y.; Ju, J.P.; Zheng, F.; Wang, T.M. High performance shape memory polyimides based on π–π interactions. J. Mater. Chem. A 2015, 3, 352–359. [Google Scholar] [CrossRef]
- Yang, Z.H.; Song, F.Z.; Wang, Q.H.; Wang, T.M. Shape Memory Induced Structural Evolution of High Performance Copolyimides. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3858–3867. [Google Scholar] [CrossRef]
- Wakita, J.; Ando, S. Characterization of Electronic Transitions in Polyimide Films Based on Spectral Variations Induced by Hydrostatic Pressures up to 400 MPa. J. Phys. Chem. B 2009, 113, 8835–8846. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshida, M.; Ando, S. Optical Properties of Rod-like Fluorinated Polyimides and Model Compounds Derived from Diamines having High Electron-donating Properties. J. Photopolym. Sci. Technol. 2006, 19, 297–304. [Google Scholar] [CrossRef]
- Takizawa, K.; Wakita, J.; Azami, S.; Ando, S. Relationship between Molecular Aggregation Structures and Optical Properties of Polyimide Films Analyzed by Synchrotron Wide-Angle X-ray Diffraction, Infrared Absorption, and UV/Visible Absorption Spectroscopy at Very High Pressure. Macromolecules 2011, 44, 349–359. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, S.Y.; Fan, L. Preparation and characterization of highly transparent and colorless semi-aromatic polyimide films derived from alicyclic dianhydride and aromatic diamines. Polymer 2012, 53, 3529–3539. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-Processable Colorless Polyimides Derived from Hydrogenated Pyromellitic Dianhydride with Controlled Steric Structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Hasegawa, M. Semi-aromatic polyimides with low dielectric constant and low CTE. High Perform. Polym. 2001, 13, S93–S106. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wang, H.M.; Chen, W.J.; Lee, T.M.; Leu, C.M. Synthesis and Properties of Novel Triptycene-Based Polyimides. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3109–3120. [Google Scholar] [CrossRef]
- Han, K.S.; You, K.; Jang, W.H.; Rhee, T.H. Synthesis and properties of chlorinated polyimides. Macromol. Chem. Phys. 2000, 201, 747–751. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Yang, C.P.; Chen, S.H. Synthesis and Properties of Ortho-Linked Aromatic Polyimides Based on 1,2-Bis(4-aminophenoxy)-4-tertbutylbenzene. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1551–1559. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.C.; Jeong, K.M.; Ando, S.; Ha, C.S. Transparent Aromatic Polyimides Derived from Thiophenyl-Substituted Benzidines with High Refractive Index and Small Birefringence. Macromolecules 2015, 48, 3462–3474. [Google Scholar] [CrossRef]
- Huang, W.; Yan, D.Y.; Lu, Q.H. Synthesis and Characterization of a Highly Soluble Aromatic Polyimide from 4,4′-Methylenebis(2-tertbutylaniline). Macromol. Rapid Commun. 2001, 22, 1481–1484. [Google Scholar] [CrossRef]
- Liaw, D.J.; Chang, F.C.; Leung, M.K.; Chou, M.Y.; Muellen, K. High Thermal Stability and Rigid Rod of Novel Organosoluble Polyimides and Polyamides Based on Bulky and Noncoplanar Naphthalene-Biphenyldiamine. Macromolecules 2005, 38, 4024–4029. [Google Scholar] [CrossRef]
- Yeo, H.; Goh, M.; Ku, B.C.; You, N.H. Synthesis and characterization of highly-fluorinated colorless polyimides derived from 4,4′-((perfluoro-[1,1′-biphenyl]-4,4′-diyl)bis(oxy))bis(2,6-dimethylaniline) and aromatic dianhydrides. Polymer 2015, 76, 280–286. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.M.; Song, G.L.; Dang, G.D.; Chen, C.H.; Zhou, H.W.; Zhao, X.G. Novel soluble polyimides derived from 2,2′-bis[4 -(5-amino-2-pyridinoxy)phenyl]hexafluoropropane: Preparation, characterization, and optical, dielectric properties. Polymer 2014, 55, 3634–3641. [Google Scholar] [CrossRef]
- Choi, C.H.; Sohn, B.H.; Chang, J.H. Colorless and transparent polyimide nanocomposites: Comparison of the properties of homo- and co-polymers. J. Ind. Eng. Chem. 2013, 19, 1593–1599. [Google Scholar] [CrossRef]
- Moon, K.H.; Chae, B.; Kim, K.S.; Lee, S.W.; Jung, Y.M. Preparation and Characterization of Transparent Polyimide–Silica Composite Films Using Polyimide with Carboxylic Acid Groups. Polymers 2019, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Huang, J.M.; Kuo, S.W.; Lu, J.S.; Chang, F.C. Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications. Polymer 2005, 46, 173–181. [Google Scholar] [CrossRef]
- Wu, S.M.; Hayakawa, T.; Kikuchi, R.; Grunzinger, S.J.; Kakimoto, M.; Oikawa, H. Synthesis and Characterization of Semiaromatic Polyimides Containing POSS in Main Chain Derived from Double-Decker-Shaped Silsesquioxane. Macromolecules 2007, 40, 5698–5705. [Google Scholar] [CrossRef]
- Devaraju, S.; Vengatesan, M.R.; Selvi, M.; Kumar, A.A.; Alagar, M. Synthesis and characterization of bisphenol-A ether diamine-based polyimide POSS nanocomposites for low K dielectric and flame-retardant applications. High Perform. Polym. 2012, 24, 85–96. [Google Scholar] [CrossRef]
- Minton, T.K.; Wright, M.E.; Tomczak, S.J.; Marquez, S.A.; Shen, L.H.; Brunsvold, A.L.; Cooper, R.; Zhang, J.M.; Vij, V.; Guenthner, A.J.; et al. Atomic Oxygen Effects on POSS Polyimides in Low Earth Orbit. ACS Appl. Mater. Interfaces 2012, 4, 492–502. [Google Scholar] [CrossRef]
- Li, X.B.; Al-Ostaz, A.; Jaradat, M.; Rahmani, F.; Nouranian, S.; Rushing, G.; Manasrah, A.; Alkhateb, H.; Finckenor, M.; Lichtenhan, J. Substantially enhanced durability of polyhedral oligomeric silsequioxane-polyimide nanocomposites against atomic oxygen erosion. J. Eur. Polym. J. 2017, 92, 233–249. [Google Scholar] [CrossRef]
- Jung, Y.; Byun, S.; Park, S.; Lee, H. Polyimide−Organosilicate Hybrids with Improved Thermal and Optical Properties. ACS Appl. Mater. Interfaces 2014, 6, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.H.; Yang, S.Y.; Hsiao, S.T.; Wang, Y.S.; Li, S.M.; Ma, C.C.M.; Tien, H.W.; Zeng, S.J. Effect of Octa(aminophenyl) Polyhedral Oligomeric Silsesquioxane Functionalized Graphene Oxide on the Mechanical and Dielectric Properties of Polyimide Composites. ACS Appl. Mater. Interfaces 2014, 6, 15802–15812. [Google Scholar] [CrossRef]
- Verker, R.; Grossman, E.; Eliaz, N. Effect of the POSS–Polyimide nanostructure on its mechanical and electrical properties. Compos. Sci. Technol. 2012, 72, 1408–1415. [Google Scholar] [CrossRef]
- Verker, R.; Grossman, E.; Gouzman, I.; Eliaz, N. TriSilanolPhenyl POSS–polyimide nanocomposites: Structure–properties relationship. Compos. Sci. Technol. 2009, 69, 2178–2184. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and Characterization of Differently Substituted Phenyl Hepta Isobutyl-Polyhedral Oligomeric Silsesquioxane/Polystyrene Nanocomposites. Polym. Compos. 2014, 35, 151–157. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Cozzo, G.; Latteri, A.; Recca, A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: Influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym. Compos. 2015, 36, 1394–1400. [Google Scholar] [CrossRef]
- Xie, F.; Huang, L.N.; Leng, J.S.; Liu, Y.J. Thermoset shape memory polymers and their composites. J. Intell. Mater. Syst. Struct. 2016, 27, 2433–2455. [Google Scholar] [CrossRef]
- Tamaki, R.; Tanaka, Y.; Asuncion, M.Z.; Choi, J.W.; Laine, R.M. Octa(aminophenyl)silsesquioxane as a Nanoconstruction Site. J. Am. Chem. Soc. 2001, 123, 12416–12417. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S.X.; Nie, K.M. Morphology and thermal properties of inorganic–organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes. Polymer 2004, 45, 5557–5568. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, I.; Bottino, F.A.; Cicala, G.; Latteri, A. Dumbbell-shaped polyhedral oligomeric silsesquioxanes/polystyrene nanocomposites: The influence of the bridge rigidity on the resistance to thermal degradation. J. Compos. Mater. 2015, 49, 2509–2517. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Hu, X.F.; Yan, J.L.; Wang, Y.X.; Mu, H.L.; Wang, Z.K.; Cheng, H.Y.; Zhao, F.Y.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Sokolowski, W.M.; Tan, S.C. Advanced Self-Deployable Structures for Space Applications. J. Spacecr. Rocket. 2007, 44, 750–754. [Google Scholar] [CrossRef]
- Liu, Y.J.; Du, H.Y.; Liu, L.W.; Leng, J.S. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Paillous, A.; Pailler, C. Degradation of multiply polymer-matrix composites induced by space environment. Composites 1994, 25, 287–295. [Google Scholar] [CrossRef]
- Wienhold, P.D.; Persons, D.F. The Development of High-Temperature Composite Solar Array Substrate Panels for the Messenger Spacecraft. SAMPE J. 2003, 39, 6–17. [Google Scholar]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 4, 20–28. [Google Scholar] [CrossRef]
- Liu, C.D.; Qin, H.H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Koerner, H.; Kelley, J.; George, J.; Drummy, L.; Mirau, P.; Bell, N.S.; Hsu, J.W.P.; Vaia, R.A. ZnO Nanorod-Thermoplastic Polyurethane Nanocomposites: Morphology and Shape Memory Performance. Macromolecules 2009, 42, 8933–8942. [Google Scholar] [CrossRef]




| Sample | OAPS (wt%) a | Tg (°C) b | Td (°C) c | Td5 (°C) d | CTE (ppm/K) e | λ0 (nm) f | T400 (%) g | T500 (%) h |
|---|---|---|---|---|---|---|---|---|
| PI-1 | 0 | 320 | 520 | 550 | 141 | 310 | 95 | 99 |
| PI-2 | 1.9 | 351 | 528 | 552 | 57 | 326 | 88 | 98 |
| PI-3 | 3.8 | 363 | 529 | 555 | 43 | 336 | 82 | 97 |
| PI-4 | 5.7 | 369 | 537 | 557 | 42 | 318 | 80 | 97 |
| PI-5 | 7.6 | 372 | 546 | 566 | 53 | 326 | 81 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Chen, X.; Zhang, X.; Zhu, C.; Yu, Y.; Wei, J. Transparent, High Glass-Transition Temperature, Shape Memory Hybrid Polyimides Based on Polyhedral Oligomeric Silsesquioxane. Polymers 2019, 11, 1058. https://doi.org/10.3390/polym11061058
Lan Z, Chen X, Zhang X, Zhu C, Yu Y, Wei J. Transparent, High Glass-Transition Temperature, Shape Memory Hybrid Polyimides Based on Polyhedral Oligomeric Silsesquioxane. Polymers. 2019; 11(6):1058. https://doi.org/10.3390/polym11061058
Chicago/Turabian StyleLan, Zhongxu, Xueli Chen, Xiao Zhang, Chongyu Zhu, Yanlei Yu, and Jia Wei. 2019. "Transparent, High Glass-Transition Temperature, Shape Memory Hybrid Polyimides Based on Polyhedral Oligomeric Silsesquioxane" Polymers 11, no. 6: 1058. https://doi.org/10.3390/polym11061058