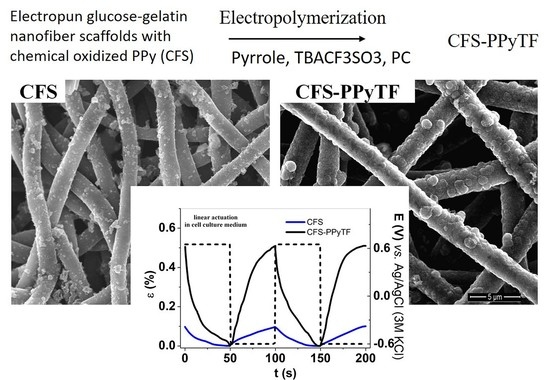
Electrochemomechanical Behavior of Polypyrrole-Coated Nanofiber Scaffolds in Cell Culture Medium
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Electropolymerization of PPy Films on Conductive Fibre Scaffolds (CFS)
2.3. Isometric and Isotonic ECMD Measurements
2.4. Characterization of samples
3. Results and Discussion
3.1. Electropolymerization, Coating Morphology
3.2. Elemental Composition
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Linear Actuation
3.4.1. Cyclic Voltammetry
3.4.2. Step Response—Efficiency and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Lee, Y.S.; Arinzeh, T.L. Electrospun nanofibrous materials for neural tissue engineering. Polymers 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Luo, Y.; Huang, J. Bio-Inspired Hierarchical Nanofibrous Fe3O4-TiO2-Carbon Composite as a High-Performance Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 17343–17351. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Nakamoto, T.; Kawazoe, N.; Chen, G. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials 2015, 73, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Siimon, K.; Siimon, H.; Järvekülg, M. Mechanical characterization of electrospun gelatin scaffolds cross-linked by glucose. J. Mater. Sci. Mater. Med. 2015, 26, 5375. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, D.; Deng, B.; Xu, X.; Nian, Q.; Jin, S.; Leedy, K.D.; Li, H.; Cheng, G.J. Flyweight, Superelastic, Electrically Conductive, and Flame-Retardant 3D Multi-Nanolayer Graphene/Ceramic Metamaterial. Adv. Mater. 2017, 29, 1605506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Yang, K.; Zhang, B.; Zhao, K.; Xiong, G.; Zhang, X. Mechanically robust and electrically conductive graphene-paper/glass-fibers/epoxy composites for stimuli-responsive sensors and Joule heating deicers. Carbon N. Y. 2017, 124, 296–307. [Google Scholar] [CrossRef]
- Xie, W.; Song, F.; Wang, R.; Sun, S.; Li, M.; Fan, Z.; Liu, B.; Zhang, Q.; Wang, J. Mechanically Robust 3D Graphene–Hydroxyapatite Hybrid Bioscaffolds with Enhanced Osteoconductive and Biocompatible Performance. Crystals 2018, 8, 105. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Sapir-Lekhovitser, Y.; Menahem, Y.; Rotenberg, J.J.; Friedman, G.; Polyak, B.; Cohen, S. Magnetically actuated tissue engineered scaffold: Insights into mechanism of physical stimulation. Nanoscale 2016, 8, 3386–3399. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yow, S.-Z.; Lim, T.H.; Yim, E.K.F.; Lim, C.T.; Leong, K.W. A 3D Electroactive Polypyrrole-Collagen Fibrous Scaffold for Tissue Engineering. Polymers 2011, 3, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Gelmi, A.; Ljunggren, M.K.; Rafat, M.; Jager, E.W.H. Influence of conductive polymer doping on the viability of cardiac progenitor cells. J. Mater. Chem. B 2014, 2, 3860. [Google Scholar] [CrossRef]
- Svennersten, K.; Berggren, M.; Richter-Dahlfors, A.; Jager, E.W.H. Mechanical stimulation of epithelial cells using polypyrrole microactuators. Lab Chip 2011, 19, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Baughman, R.H. Conducting polymer artificial muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Hsu, C.F.; Zhang, L.; Peng, H.; Travas-Sejdic, J.; Kilmartin, P.A. Scavenging of DPPH free radicals by polypyrrole powders of varying levels of overoxidation and/or reduction. Synth. Met. 2008, 158, 946–952. [Google Scholar] [CrossRef]
- Põldsalu, I.; Rohtlaid, K.; Nguyen, T.M.G.; Plesse, C.; Vidal, F.; Khorram, M.S.; Peikolainen, A.L.; Tamm, T.; Kiefer, R. Thin ink-jet printed trilayer actuators composed of PEDOT:PSS on interpenetrating polymer networks. Sens. Actuators B Chem. 2018, 258, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Artificial muscles: A tool to quantify exchanged solvent during biomimetic reactions. Chem. Mater. 2012, 24, 4093–4099. [Google Scholar] [CrossRef]
- Pyo, M.; Bohn, C.C.; Smela, E.; Reynolds, J.R.; Brennan, A.B. Direct strain measurement of polypyrrole actuators controlled by the polymer/gold interface. Chem. Mater. 2003, 15, 916–922. [Google Scholar] [CrossRef]
- Kiefer, R.; Chu, S.Y.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Mixed-ion linear actuation behaviour of polypyrrole. Electrochim. Acta 2007, 52, 2386–2391. [Google Scholar] [CrossRef]
- Jaakson, P.; Aabloo, A.; Tamm, T. Encapsulation of ionic electroactive polymers: Reducing the interaction with environment. In Proceedings of the SPIE, San Jose, CA, USA, 21–25 February 2016; Volume 9798, p. 979825. [Google Scholar]
- Siimon, K.; Reemann, P.; Pōder, A.; Pook, M.; Kangur, T.; Kingo, K.; Jaks, V.; Möeorg, U.; Järvekülg, M. Effect of glucose content on thermally cross-linked fibrous gelatin scaffolds for tissue engineering. Mater. Sci. Eng. C 2014, 42, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Harjo, M.; Kesküla, A.; Leemets, K.; Khorram, M.S.; Saar, R.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole coatings on gelatin fiber scaffolds: Material and electrochemical characterizations in organic and aqueous electrolyte. Synth. Met. 2017, 232, 25–30. [Google Scholar] [CrossRef]
- Kivilo, A.; Zondaka, Z.; Kesküla, A.; Rasti, P.; Tamm, T.; Kiefer, R. Electro-chemo-mechanical deformation properties of polypyrrole/dodecylbenzenesulfate linear actuators in aqueous and organic electrolyte. RSC Adv. 2016, 6, 69–75. [Google Scholar] [CrossRef]
- Kiefer, R.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Actuation of polypyrrole films in propylene carbonate electrolytes. Sens. Actuators B Chem. 2007, 125, 628–634. [Google Scholar] [CrossRef]
- Raudsepp, T.; Marandi, M.; Tamm, T.; Sammelselg, V.; Tamm, J. Influence of ion-exchange on the electrochemical properties of polypyrrole films. Electrochim. Acta 2014, 122, 79–86. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Lee, B.-T. Fabrication and characterization of cross-linked gelatin electro-spun nano-fibers. J. Biomed. Sci. Eng. 2010, 3, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, G.; Li, C.; Liang, Y. Thin polypyrrole films prepared by chemical oxidative polymerization. J. Appl. Polym. Sci. 1998, 70, 2169–2172. [Google Scholar] [CrossRef]
- Marcilla, R.; Pozo-Gonzalo, C.; Rodríguez, J.; Alduncin, J.A.; Pomposo, J.A.; Mecerreyes, D. Use of polymeric ionic liquids as stabilizers in the synthesis of polypyrrole organic dispersions. Synth. Met. 2006, 156, 1133–1138. [Google Scholar] [CrossRef]
- Tamm, J.; Johanson, U.; Marandi, M.; Tamm, T. Influence of anions on electrochemical properties of polypyrrole films. Russ. J. Electrochem. 1998, 1, 51014. [Google Scholar]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G. Ionic exchanges, structural movements and driven reactions in conducting polymers from bending artificial muscles. Sens. Actuators B Chem. 2014, 199, 27–30. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250–251, 167–174. [Google Scholar] [CrossRef] [PubMed]






| Inorganic Salts | Concentration [g L−1] |
|---|---|
| NaHCO3 | 3.7 |
| NaCl | 6.4 |
| CaCl2 | 0.2 |
| MgSO4 | 0.097 |
| NaH2PO4 | 0.109 |
| KCl | 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harjo, M.; Torop, J.; Järvekülg, M.; Tamm, T.; Kiefer, R. Electrochemomechanical Behavior of Polypyrrole-Coated Nanofiber Scaffolds in Cell Culture Medium. Polymers 2019, 11, 1043. https://doi.org/10.3390/polym11061043
Harjo M, Torop J, Järvekülg M, Tamm T, Kiefer R. Electrochemomechanical Behavior of Polypyrrole-Coated Nanofiber Scaffolds in Cell Culture Medium. Polymers. 2019; 11(6):1043. https://doi.org/10.3390/polym11061043
Chicago/Turabian StyleHarjo, Madis, Janno Torop, Martin Järvekülg, Tarmo Tamm, and Rudolf Kiefer. 2019. "Electrochemomechanical Behavior of Polypyrrole-Coated Nanofiber Scaffolds in Cell Culture Medium" Polymers 11, no. 6: 1043. https://doi.org/10.3390/polym11061043