Improving the Damping Properties of Nanocomposites by Monodispersed Hybrid POSS Nanoparticles: Preparation and Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
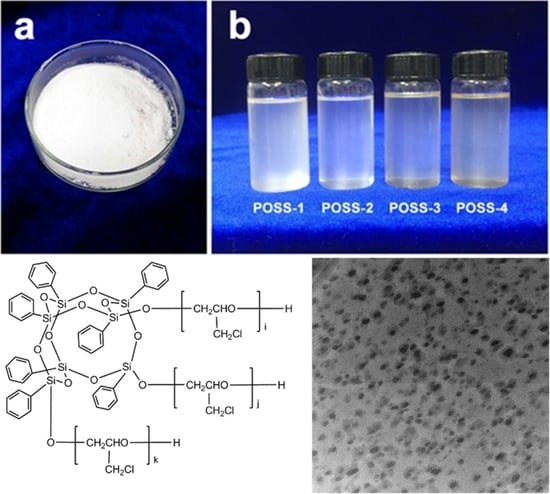
2.2. Synthesis of POSS–PECH
2.3. Sample Preparation
2.4. Gel Extraction Experiments
2.5. Characterization
3. Results
3.1. Chemical Structural Analysis of POSS–PECH
3.2. Structure and Phase Morphology Analysis of POSS-Based Polyurethane Nanocomposites
3.3. Damping Properties
3.3.1. Effect of POSS1 Content on the Damping Properties of PU Composites
3.3.2. Effect of Molecular Weight Change of POSS–PECH on Damping Property of Composites
3.3.3. Effect of R Value on Damping Property of Polyurethane Polymer
3.3.4. Effect of Different Testing Frequencies on Damping Property of Polyurethane Polymer
4. Discussion
4.1. Analysis of the Mechanism(s) of Tg and T1 Changes in POSS-Based Polyurethane Nanocomposites
4.2. Analysis of the Damping Mechanism of the POSS-Based Polyurethane Composite System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tjong, S.C. Structural and mechanical properties of polymernanocomposites. Mater. Sci. Eng. R. Rep. 2006, 53, 72–197. [Google Scholar] [CrossRef]
- Krishnamoorti, R.; Vaia, R.A. Polymer nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3252–3256. [Google Scholar] [CrossRef] [Green Version]
- Meszaros, L. Polymer matrix hybrid composites: The efficient way of improved performance. Express Polym. Lett. 2014, 8, 790. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.J.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation &functionalization with nanocarbons. Prog. Polym. Sc. 2018, 86, 40–84. [Google Scholar]
- Machrafi, H.; Lebon, G.; Iorio, C.S. Effect of volume-fraction dependent agglomeration of nanoparticles on the thermal conductivity of nanocomposites: Applications to epoxy resins, filled by SiO2, AlN and MgO nanoparticles. Compos. Sci. Technol. 2016, 130, 78–87. [Google Scholar] [CrossRef]
- Lee, D.W.; Yoo, B.R. Advanced silica/polymer composites: Materials and applications. J. Ind. Eng. Chem. 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Yu, D.Y.; Shao, X.Y.; Zhang, S.Q.; Wang, S. Research and applications of viscoelastic vibration damping materials: A review. Compos. Struct. 2016, 136, 460–480. [Google Scholar] [CrossRef]
- Sun, L.Y.; Gibson, R.F.; Gordaninejad, F.; Suhr, J. Energy absorption capability of nanocomposites: A review. Compos. Sci. Technol. 2009, 69, 2392–2409. [Google Scholar] [CrossRef]
- Zhu, G.L.; Han, D.; Yuan, Y.; Chen, F.; Fu, Q. Improving Damping Properties and Thermal Stability of Epoxy/Polyurethane Grafted Copolymer by Adding Glycidyl POSS. Chin. J. Polym. Sci. 2018, 36, 1297–1302. [Google Scholar] [CrossRef]
- Ristić, I.S.; Simendić, J.B.; Krakovsky, I.; Valentova, H.; Radičević, R.; Cakić, S.; Nikolić, N. The properties of polyurethane hybrid materials based on castor oil. Chin. Mater. Chem. Phys. 2012, 132, 74–81. [Google Scholar] [CrossRef]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Shear Strength and Morphological Study of Polyurethane-OMMT Clay Nanocomposite Adhesive Derived from Vegetable Oil-Based Constituents. J. Renew. Mater. 2018, 6, 117–125. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, H.Y.; Pan, G.Q. Mechanical and damping properties of carbon nanotube-modified polyisobutylene-based polyurethane composites. J. Compos. Mater. 2016, 50, 929–936. [Google Scholar] [CrossRef]
- Michael, E.M.; Anish, T.; Phillip, M.D. General strategies for nanoparticle dispersion. Science 2006, 311, 1740–1743. [Google Scholar]
- Gupta, S.; Zhang, Q.L.; Emrick, T. Entropy-driven segregation of nanoparticles to cracks in multilayered composite polymer structures. Nat. Mater. 2006, 5, 229–233. [Google Scholar] [CrossRef]
- Anna, C.B.; Emrick, T.; Russell, T.P. Nanoparticle polymer compositesn: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar]
- Guo, Z.R.; Chang, T.C.; Guo, X.M. Thermal-Induced Edge Barriers and Forces in Interlayer Interaction of Concentric Carbon Nanotubes. Phys. Rev. Lett. 2011, 107, 105502. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.C.; Zhang, H.W.; Guo, Z.R. Nanoscale directional motion towards regions of higher stiffness. Phys. Rev. Lett. 2015, 114, 015504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Zhang, H.W.; Guo, Z.R.; Chang, T.C. Edge Forces in Contacting Graphene Layers. J. Appl. Mech. 2015, 82, 101011. [Google Scholar] [CrossRef]
- Suhr, J.; Koratkar, N.; Keblinski, P.; Ajayan, P. Viscoelasticity in carbon nanotube composites. Compos. Sci. Technol. 2005, 4, 134–137. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Wang, T. Damping, thermal, and mechanical properties of carbon nanotubes modified castor oilbased polyurethane/epoxy interpenetrating polymer network composites. Mater. Design. 2012, 38, 47–52. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Wang, T. Damping, thermal, and mechanical properties of montmorillonite modified castor oilbased polyurethane/epoxy graft IPN composites. Mater. Chem. Phys. 2011, 130, 680–684. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Wang, T.; Pei, X. Preparation, damping and thermal properties of potassium titanate whiskers filled castor oil-based polyurethane/epoxy interpenetrating polymer network composites. Compos. Sci. Technol. 2011, 32, 803–807. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Cozzo, G.; Latteri, A.; Recca, A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: Influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym. Compos. 2014, 36, 1394–1400. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Tanaka, K.; Adachi, S.; Chujo, Y. Structure-property relationship of octa-substituted POSS in thermal and mechanical reinforcements of conventional polymers. J. Polym. Sci. 2009, 47, 5690–5697. [Google Scholar] [CrossRef]
- Trakulsujaritchok, T.; Hourston, D.J. Damping characteristics and mechanical properties of silica Wlled PUR/PEMA simultaneous interpenetrating polymer networks. Eur. Pol. J. 2006, 42, 2968–2976. [Google Scholar] [CrossRef]
- Zhang, H.W.; Wang, B.; Li, H.T.; Yan, J.; Wang, J.Y. Synthesis and characterization of nanocomposites of silicon dioxide and polyurethane and epoxy resin interpenetrating network. Polym. Int. 2003, 52, 1493–1497. [Google Scholar] [CrossRef]
- Robert, M.E.; Tyler, R.L.; Erich, D.B.; Joseph, L.L.; Timothy, W.S. Mechanics and nanovoid nucleation dynamics: Effects of polar functi- onality in glassy polymer networks. Soft Matter 2018, 14, 8895–8911. [Google Scholar]
- Fu, Z. Strength theory of polymer materials. In Strength and Failure Behavior of Polymer Materials, 1st ed.; Chemical Industry Press: Beijing China, 2005; pp. 41–42. [Google Scholar]






| PECH Samples | T7-POSS (mol) | ECH (mol) | BF3·OEt2 (mol) | Mw of POSS–PECH |
|---|---|---|---|---|
| POSS-1 | 1 | 10 | 0.6 | 1573 |
| POSS-2 | 1 | 25 | 0.6 | 2762 |
| POSS-3 | 1 | 40 | 0.6 | 3533 |
| POSS-4 | 1 | 55 | 0.6 | 4136 |
| PU Samples | Castor Oil (g) | PECH (g) | POSS1 (g) | POSS2 (g) | POSS3 (g) | POSS4 (g) | PAPI (g) |
|---|---|---|---|---|---|---|---|
| Pure-PECH/PU | 10 | 10 | 6.09 | ||||
| 1.75%-POSS1/PU | 10 | 10 | 2 | 6.28 | |||
| 4.54%-POSS1/PU | 10 | 10 | 6 | 6.66 | |||
| 7.57%-POSS1/PU | 10 | 10 | 12 | 7.22 | |||
| 9.72%-POSS1/PU | 10 | 10 | 18 | 7.79 | |||
| 5.55%-POSS2/PU | 10 | 10 | 18 | 7.68 | |||
| 4.35%-POSS3/PU | 10 | 10 | 18 | 7.60 | |||
| 3.74%-POSS4/PU | 10 | 10 | 18 | 7.48 | |||
| POSS1/PU-0.90 | 10 | 10 | 18 | 6.67 | |||
| POSS1/PU-0.95 | 10 | 10 | 18 | 7.05 | |||
| POSS1/PU-1.00 | 10 | 10 | 18 | 7.42 | |||
| POSS1/PU-1.05 | 10 | 10 | 18 | 7.79 |
| PECH Samples | Mp | Mn | Mv | Mw | Mz | Mz + 1 | PD |
|---|---|---|---|---|---|---|---|
| POSS-1 | 1607 | 1348 | 1538 | 1573 | 1812 | 2057 | 1.1669 |
| POSS-2 | 2120 | 1960 | 2628 | 2762 | 3737 | 4698 | 1.4092 |
| POSS-3 | 3841 | 2439 | 3365 | 3533 | 4689 | 5730 | 1.4485 |
| POSS-4 | 4871 | 2614 | 3894 | 4136 | 5849 | 7420 | 1.5822 |
| PECH Samples | Hydroxyl Value (mol/g) |
|---|---|
| POSS-1 | 6.64 × 10−4 |
| POSS-2 | 6.20 × 10−4 |
| POSS-3 | 5.90 × 10−4 |
| POSS-4 | 5.42 × 10−4 |
| PU Samples | Inorganic Core Content (%) | tan δ Tg/°C | Damping Temperature Field (tan δ > 0.3) | |||
|---|---|---|---|---|---|---|
| T1/°C | T2/°C | ΔT°C | ||||
| Pure-PECH/PU | 0 | 0.9064 | 15.8 | -1.1 | 33.3 | 34.4 |
| 1.75%-POSS1/PU | 1.75 | 0.9659 | 14.0 | -2.0 | 32.6 | 34.6 |
| 4.54%-POSS1/PU | 4.54 | 1.058 | 14.1 | -2.7 | 34.2 | 36.9 |
| 7.57%-POSS1/PU | 7.57 | 1.099 | 12.3 | -4.0 | 37.4 | 41.4 |
| 9.72%-POSS1/PU | 9.72 | 1.164 | 9.4 | -6.9 | 37.1 | 44.0 |
| PU Samples | Inorganic Core Content (%) | tan δ Tg/°C | Damping Temperature Field (tan δ > 0.3) | |||
|---|---|---|---|---|---|---|
| T1/°C | T2/°C | ΔT°C | ||||
| Pure PECH/PU 9.72%-POSS1/PU | 0 9.72 | 0.964 1.164 | 15.8 9.4 | −1.1 −6.9 | 33.3 37.1 | 34.4 44.0 |
| 5.55%-POSS2/PU | 5.55 | 1.091 | 6.5 | −10.3 | 34.0 | 44.3 |
| 4.35%-POSS3/PU | 4.35 | 0.9813 | 3.7 | −15.1 | 28.2 | 43.3 |
| 3.74%-POSS4/PU | 3.74 | 0.9681 | 0.6 | −18.1 | 25.9 | 44.0 |
| PU Samples [POSS/PU–R] | Inorganic Core Content (%) | tan δ Tg/°C | Damping Temperature Field (tan δ > 0.3) | |||
|---|---|---|---|---|---|---|
| T1/°C | T2/°C | ΔT°C | ||||
| POSS1/PU-0.90 | 9.96 | 1.186 | 7.5 | −12.3 | 35.8 | 48.1 |
| POSS1/PU-0.95 | 9.88 | 1.201 | 9.6 | −10.4 | 33.6 | 44.0 |
| POSS1/PU-1.00 | 9.80 | 1.262 | 9.6 | −9.7 | 35.1 | 44.8 |
| POSS1/PU-1.05 | 9.72 | 1.164 | 9.4 | −6.9 | 37.1 | 44.0 |
| Test Frequency (Hz) | Tan δ Tg/°C | Damping Temperature Field (tan δ > 0.3) | |||
|---|---|---|---|---|---|
| T1/°C | T2/°C | ΔT°C | |||
| 0.5 | 1.234 | 9.9 | −12.3 | 34.5 | 46.8 |
| 1 | 1.262 | 9.6 | −9.7 | 35.1 | 44.8 |
| 3 | 1.111 | 10.7 | −8.7 | 40.1 | 48.8 |
| 5 | 1.119 | 14.1 | −6.8 | 48.5 | 55.3 |
| PU Samples | tan δ Tg/°C | Damping Temperature Field (tan δ > 0.3) | |||
|---|---|---|---|---|---|
| T1/°C | T2/°C | ΔT°C | |||
| pure-PECH/PU | 0.9064 | 15.75 | −1.1 | 33.3 | 34.4 |
| 1.96%-SiO2/PU | 0.8319 | 15.70 | −0.8 | 34.8 | 35.6 |
| 4.75%-SiO2/PU | 0.7729 | 17.50 | 0.2 | 34.8 | 34.6 |
| 7.59%-SiO2/PU | 0.7065 | 18.25 | 1.2 | 36.8 | 35.6 |
| 9.74%-SiO2/PU | 0.6761 | 21.00 | 4.4 | 38.7 | 34.3 |
| PU Samples | Tensile Strength (MPa) | Modulus of Elasticity (MPa) | Elongation at Break (%) | Critical Fracture Stress (MPa) | Cohesive Energy Density (MPa) | Gel Content (%) |
|---|---|---|---|---|---|---|
| Pure-PECH/PU | 8.32 | 10.90 | 142.5 | 8.32 | 0.82 | 96.28 |
| 1.75%-POSS1/PU | 8.18 | 9.97 | 160 | 8.18 | 0.75 | 90.20 |
| 4.54%-POSS1/PU | 5.70 | 7.54 | 140 | 7.86 | 0.57 | 83.18 |
| 7.57%-POSS1/PU | 4.84 | 6.26 | 150 | 5.21 | 0.47 | 78.47 |
| 9.72%-POSS1/PU | 3.89 | 5.29 | 145 | 3.89 | 0.40 | 70.10 |
| PU Samples | Tensile Strength (MPa) | Modulus of Elasticity (MPa) | Elongation at Break (%) | Critical Fracture Stress (MPa) | Cohesive Energy Density (MPa) | Gel Content (%) |
|---|---|---|---|---|---|---|
| Pure-PECH/PU | 8.32 | 10.90 | 142.5 | 8.32 | 0.82 | 96.28 |
| 1.96%-SiO2/PU | 5.76 | 12.18 | 94.0 | 5.76 | 0.92 | 98.24 |
| 4.75%-SiO2/PU | 6.87 | 17.00 | 86.7 | 6.87 | 1.28 | 96.67 |
| 7.59%-SiO2/PU | 10.21 | 23.93 | 84.7 | 10.21 | 1.80 | 97.39 |
| 9.74%-SiO2/PU | 15.83 | 31.93 | 102 | 15.83 | 2.40 | 96.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Zhang, Y.; Liu, M.; Zhang, Y.; Yin, Y.; Gutowski, W.S.; Deng, P.; Zheng, C. Improving the Damping Properties of Nanocomposites by Monodispersed Hybrid POSS Nanoparticles: Preparation and Mechanisms. Polymers 2019, 11, 647. https://doi.org/10.3390/polym11040647
Wei W, Zhang Y, Liu M, Zhang Y, Yin Y, Gutowski WS, Deng P, Zheng C. Improving the Damping Properties of Nanocomposites by Monodispersed Hybrid POSS Nanoparticles: Preparation and Mechanisms. Polymers. 2019; 11(4):647. https://doi.org/10.3390/polym11040647
Chicago/Turabian StyleWei, Wei, Yingjun Zhang, Meihua Liu, Yifan Zhang, Yuan Yin, Wojciech Stanislaw Gutowski, Pengyang Deng, and Chunbai Zheng. 2019. "Improving the Damping Properties of Nanocomposites by Monodispersed Hybrid POSS Nanoparticles: Preparation and Mechanisms" Polymers 11, no. 4: 647. https://doi.org/10.3390/polym11040647