Synthesis and Characterization of 3-DOM IrO2 Electrocatalysts Templated by PMMA for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of PMMA Templates
2.2. Preparation of 3-DOM IrO2
2.3. Physical Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
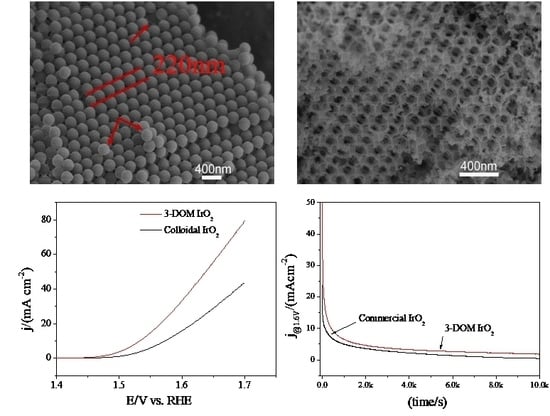
3.1. Physical Characterization
3.2. Electrocatalytic Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bockris, J.O.M. The hydrogen economy: Its history. Int. J. Hydrogen Energy 2013, 38, 2579–2588. [Google Scholar]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Ma, H.; Zhong, H.; Zou, Y. Study of carbon-supported IrO2 and RuO2 for use in the hydrogen evolution reaction in a solid polymer electrolyte electrolyzer. Electrochim. Acta 2010, 55, 1855–1861. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Yang, H.N.; Park, S.H.; Lee, D.C.; Yi, S.C.; Kim, W.J. Electrochemical properties of hybrid typed electrocatalyst using Pt/carbon molecular sieve synthesized by zeolite template and Pt carbon black. Microporous Mesoporous Mater. 2013, 172, 161–166. [Google Scholar] [CrossRef]
- Kaewsai, D.; Yeamdee, S.; Supajaroon, S.; Hunsom, M. Orr activity and stability of PtCr/C catalysts in a low temperature/pressure PEM fuel cell: Effect of heat treatment temperature. Int. J. Hydrogen Energy 2018, 43, S0360319918301769. [Google Scholar] [CrossRef]
- Cantane, D.A.; Oliveira, F.E.R.; Santos, S.F.; Lima, F.H.B. Synthesis of Pt-based hollow nanoparticles using carbon-supported Co@Pt and Ni@Pt core–shell structures as templates: Electrocatalytic activity for the oxygen reduction reaction. Appl. Catal. B Environ. 2013, 136–137, 351–360. [Google Scholar] [CrossRef]
- Yun, Y.H.; Kim, S.D.; Park, S.W.; Lee, J.Y.; Yi, S.C.; Kim, W.J. Cell performance of MEA fabricated with Pt-ZSM-5-carbon electrode for PEMFC. Microporous Mesoporous Mater. 2010, 131, 122–127. [Google Scholar] [CrossRef]
- Kishor, R.; Singh, S.B.; Ghoshal, A.K. Role of metal type on mesoporous KIT-6 for hydrogen storage. Int. J. Hydrogen Energy 2018, S0360319918312643. [Google Scholar] [CrossRef]
- Alam, N.; Mokaya, R. Characterisation and hydrogen storage of Pt-doped carbons templated by Pt-exchanged zeolite y. Microporous Mesoporous Mater. 2011, 142, 716–724. [Google Scholar] [CrossRef]
- Miles, M.H.; Klaus, E.A.; Gunn, B.P.; Locker, J.R.; Serafin, W.E.; Srinivasan, S. The oxygen evolution reaction on platinum, iridium, ruthenium and their alloys at 80 °C in acid solutions. Electrochim. Acta 1978, 23, 521–526. [Google Scholar] [CrossRef]
- Benedetti, A.; Riello, P.; Battaglin, G.; Battisti, A.D.; Barbieri, A. Physicochemical properties of thermally prepared Ti-supported IrO2 + ZrO2 electrocatalysts. J. Electroanal. Chem. 1994, 376, 195–202. [Google Scholar] [CrossRef]
- Lu, Z.-X.; Shi, Y.; Yan, C.-F.; Guo, C.-Q.; Wang, Z.-D. Investigation on IrO2 supported on hydrogenated TiO2 nanotube array as OER electro-catalyst for water electrolysis. Int. J. Hydrogen Energy 2017, 42, 3572–3578. [Google Scholar] [CrossRef]
- Nikolić, B.Ž.; Panić, V.V.; Dekanski, A.B. Intrinsic potential-dependent performances of a Sol–Gel-Prepared electrocatalytic IrO2–TiO2 coating of dimensionally stable anodes. Electrocatalysis 2012, 3, 360–368. [Google Scholar] [CrossRef]
- Osman, J.R.; Crayston, J.A.; Pratt, A.; Richens, D.T. Sol–gel processing of IrO2–TiO2 mixed metal oxides based on a hexachloroiridate precursor. J. Sol-Gel Sci. Technol. 2007, 44, 219–225. [Google Scholar] [CrossRef]
- Oakton, E.; Lebedev, D.; Povia, M.; Abbott, D.F.; Fabbri, E.; Fedorov, A.; Nachtegaal, M.; Copéret, C.; Schmidt, T.J. IrO2-TiO2: A high-surface-area, active, and stable electrocatalyst for the oxygen evolution reaction. ACS Catal. 2017, 7, 2346–2352. [Google Scholar] [CrossRef]
- Nikiforov, A.V.; García, A.L.T.; Petrushina, I.M.; Christensen, E.; Bjerrum, N.J. Preparation and study of IrO2/SiC-Si supported anode catalyst for high temperature pem steam electrolysers. Int. J. Hydrogen Energy 2011, 36, 5797–5805. [Google Scholar] [CrossRef]
- Xu, W.; Tayal, J.; Basu, S.; Scott, K. Nano-crystalline RuxSn1-xO2 powder catalysts for oxygen evolution reaction in proton exchange membrane water electrolysers. Int. J. Hydrogen Energy 2011, 36, 14796–14804. [Google Scholar]
- Ferro, S.; Rosestolato, D.; Martínez-Huitle, C.A.; Battisti, A.D. On the oxygen evolution reaction at IrO2-SnO2 mixed-oxide electrodes. Electrochim. Acta 2014, 146, 257–261. [Google Scholar] [CrossRef]
- Xu, J.; Liu, G.; Li, J.; Wang, X. The electrocatalytic properties of an IrO2/SnO2 catalyst using SnO2 as a support and an assisting reagent for the oxygen evolution reaction. Electrochim. Acta 2012, 59, 105–112. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Hao, J.; Chi, J.; Fan, Z.; Yao, D.; Qin, B.; Shao, Z. An effective oxygen electrode based on Ir0.6Sn0.4O2 for pem water electrolyzers. J. Energy Chem. 2019, 39, 23–28. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Feng, Z.; Tang, M.; Yuan, X.; Tan, Z. Promotion of in situ tin x interlayer on morphology and electrochemical properties of titanium based IrO2-Ta2O5 coated anode. J. Alloys Compd. 2017, 708, 1081–1088. [Google Scholar] [CrossRef]
- Cuevas, O.; Herrada, R.A.; Corona, J.L.; Olvera, M.G.; Sepúlveda-Guzmán, S.; Sirés, I.; Bustos, E. Assessment of IrO2-Ta2O5|Ti electrodes for the electrokinetic treatment of hydrocarbon-contaminated soil using different electrode arrays. Electrochim. Acta 2016, 208, 282–287. [Google Scholar] [CrossRef]
- Kadakia, K.S.; Jampani, P.H.; Velikokhatnyi, O.I.; Datta, M.K.; Patel, P.; Chung, S.J.; Park, S.K.; Poston, J.A.; Manivannan, A.; Kumta, P.N. Study of fluorine doped (Nb, Ir)O2 solid solution electro-catalyst powders for proton exchange membrane based oxygen evolution reaction. Mater. Sci. Eng. B 2016, 212, 101–108. [Google Scholar] [CrossRef]
- El Sawy, E.N.; Birss, V.I. Nano-porous iridium and iridium oxide thin films formed by high efficiency electrodeposition. J. Mater. Chem. 2009, 19, 8244–8252. [Google Scholar] [CrossRef]
- Cherevko, S.; Reier, T.; Zeradjanin, A.R.; Pawolek, Z.; Strasser, P.; Mayrhofer, K.J.J. Stability of nanostructured iridium oxide electrocatalysts during oxygen evolution reaction in acidic environment. Electrochem. Commun. 2014, 48, 81–85. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Nanoporous IrO2 catalyst with enhanced activity and durability for water oxidation owing to its micro/mesoporous structure. Nanoscale 2017, 9, 9291. [Google Scholar] [CrossRef]
- Ortel, E.; Reier, T.; Strasser, P.; Kraehnert, R. Mesoporous IrO2 films templated by PEO-PB-PEO Block-Copolymers: Self-Assembly, Crystallization Behavior, and Electrocatalytic performance. Chem. Mater. 2011, 23, 3201–3209. [Google Scholar] [CrossRef]
- Lei, W.; Wang, F.; Zhu, J.; Xin, Z.; Yi, T.; Xing, W. Synthesis and electrochemical performance of three-dimensional ordered hierarchically porous Li4Ti5O12 for high performance lithium ion batteries. Ceram. Int. 2017, 44, S0272884217317418. [Google Scholar]
- Ergang, N.S.; Lytle, J.C.; Lee, K.T.; Oh, S.M.; Smyrl, W.H.; Stein, A. Photonic crystal structures as a basis for a Three-Dimensionally interpenetrating electrochemical-cell system. Adv. Mater. 2010, 18, 1750–1753. [Google Scholar] [CrossRef]
- Chunzhen, Y.; Ming, Z.; Qian, X. Three-dimensional ordered macroporous MnO2/carbon nanocomposites as high-performance electrodes for asymmetric supercapacitors. Phys. Chem. Chem. Phys. 2013, 15, 19730–19740. [Google Scholar]
- Hu, W.; Wang, Y.; Hu, X.; Zhou, Y.; Chen, S. Three-dimensional ordered macroporous IrO2 as electrocatalyst for oxygen evolution reaction in acidic medium. J. Mater. Chem. 2012, 22, 6010. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, P.; Xu, S.; Chen, S.; Xia, Q. Template synthesis of 3-DOM IrO2 powder catalysts: Temperature-dependent pore structure and electrocatalytic performance. J. Mater. Sci. 2015, 50, 2984–2992. [Google Scholar] [CrossRef]
- Haddadine, N.; Agoudjil, K.; Abouzeid, K.; Castano, C.E.; Bouslah, N.; Benaboura, A.; Samy El-Shall, M. Optical and physical properties of iridescent photonic crystals obtained by self-assembled polymethyl methacrylate nanospheres within graphene oxide nanoplatelets. Polym. Adv. Technol. 2018, 29, 244–253. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, P.; Chmelka, B.F.; Stucky, G.D. Multiphase assembly of Mesoporous-Macroporous Membranes. Chem. Mater. 1999, 11, 1174–1178. [Google Scholar] [CrossRef]
- Woods, R. Hydrogen adsorption on platinum, iridium and rhodium electrodes at reduced temperatures and the determination of real surface area. J. Electroanal. Chem. Interfacial Electrochem. 1974, 49, 217–226. [Google Scholar] [CrossRef]
- Papaderakis, A.; Tsiplakides, D.; Balomenou, S.; Sotiropoulos, S. Probing the hydrogen adsorption affinity of Pt and Ir by surface interrogation scanning electrochemical microscopy (si-secm). Electrochem. Commun. 2017, 83, 77–80. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Sun, X.; Chen, X.; Li, C.; Yu, J.; Tang, H. Synthesis and Characterization of 3-DOM IrO2 Electrocatalysts Templated by PMMA for Oxygen Evolution Reaction. Polymers 2019, 11, 629. https://doi.org/10.3390/polym11040629
Liu F, Sun X, Chen X, Li C, Yu J, Tang H. Synthesis and Characterization of 3-DOM IrO2 Electrocatalysts Templated by PMMA for Oxygen Evolution Reaction. Polymers. 2019; 11(4):629. https://doi.org/10.3390/polym11040629
Chicago/Turabian StyleLiu, Fei, Xuechu Sun, Xiu Chen, Cuicui Li, Jun Yu, and Haolin Tang. 2019. "Synthesis and Characterization of 3-DOM IrO2 Electrocatalysts Templated by PMMA for Oxygen Evolution Reaction" Polymers 11, no. 4: 629. https://doi.org/10.3390/polym11040629