Anchor Effect in Polymerization Kinetics: Case of Monofunctionalized POSS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
1M-POSS Preparation
2.2. Methods
2.2.1. Viscosity
2.2.2. Glass Transition
2.2.3. NMR Study
2.2.4. Photopolymerization Kinetics
2.2.5. Photorheology
2.2.6. GPC
2.2.7. TEM
3. Results and Discussion
3.1. Viscosity of Formulations
3.2. NMR Analysis
3.3. Photopolymerization Kinetics
3.3.1. DSC Study
3.3.2. Photorheological Study
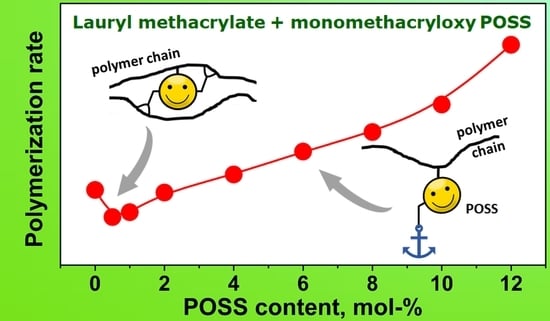
3.3.3. Polymerization Rate Coefficients
3.4. Copolymer Characterization
3.4.1. GPC Analysis
3.4.2. TEM Analysis
3.5. Glass Transition Temperature, Tg
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhong, W. PMMA-, PAN-, and Acrylic-Based Polymer Nanocomposites. In Recent Advances in Polymer Nano-composites; Thomas, S., Zaikov, G.E., Valsaraj, S.V., Eds.; CRC Press: Leiden, The Netherlands; Boston, MA, USA, 2009; pp. 155–181. [Google Scholar]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Blanco, I. The rediscovery of POSS: A molecule rather than a filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. (Ed.) Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: New York, NY, USA, 2011. [Google Scholar]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral Oligomeric Silsesquioxanes (POSS)-Containing Nanohybrid Polymers. Adv. Polym. Sci. 2006, 201, 225–296. [Google Scholar] [CrossRef]
- Prządka, D.; Andrzejewska, E.; Marcinkowska, A. Multimethacryloxy-POSS as a crosslinker for hydrogel materials. Eur. Polym. J. 2015, 72, 34–49. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photoinitiated polymerization in ionic liquids and its application. Polym. Int. 2017, 66, 366–381. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Huang, L.; Gao, Y.; Ban, J.; Shen, L.; Chen, J. Structure-property relationships in hybrid dental nanocomposite resins containing monofunctional and multifunctional polyhedral oligomeric silsesquioxanes. Int. J. Nanomed. 2014, 9, 841–852. [Google Scholar] [CrossRef]
- Amerio, E.; Sangermano, M.; Colucci, G.; Malucelli, G.; Messori, M.; Taurino, R.; Fabbri, P. UV curing of organic-inorganic hybrid coatings containing polyhedral oligomeric silsesquioxane blocks. Macromol. Mater. Eng. 2008, 293, 700–707. [Google Scholar] [CrossRef]
- Fong, H.; Dickens, S.H.; Flaim, G.M. Evaluation of dental restorative composites containing polyhedral oligomeric silsesquioxane methacrylate. Dent. Mater. 2005, 21, 520–529. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Xue, X. Synthesis and characterization of UV-cured epoxy acrylate/POSS nanocomposites. Prog. Org. Coat. 2013, 76, 863–869. [Google Scholar] [CrossRef]
- Florea, N.M.; Damian, C.M.; Ionescu, C.; Lungu, A.; Vasile, E.; Iovu, H. Designing of polyhedral oligomeric silsesquioxane (POSS)-based dithiol/dimethacrylate nano-hybrids. Polym. Bull. 2018, 75, 3897–3916. [Google Scholar] [CrossRef]
- Lungu, A.; Florea, N.M.; Iovu, H. Dimethacrylic/epoxy interpenetrating polymer networks including octafunctional POSS. Polymer 2012, 53, 300–307. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Xue, X. Morphology and properties of UV-curing epoxy acrylate coatings modified with methacryl-POSS. Prog. Org. Coat. 2015, 78, 404–410. [Google Scholar] [CrossRef]
- Norouzi, S.; Mohseni, M.; Yahyaei, H. Preparation and characterization of an acrylic acid modified polyhedral oligomeric silsesquioxane and investigating its effect in a UV curable coating. Prog. Org. Coat. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Matejka, L. Epoxy-silica/silsesquioxane nanocomposites. In Hybrid Nanocomposites for Nanotechnology. Electronic, Optical, Magnetic and Biomedical Applications; Merhari, L., Ed.; Springer Science & Business Media LLC.: New Delhi, India, 2009. [Google Scholar]
- Romo–Uribe, A.; Mather, P.T.; Haddad, T.S.; Lichtenhan, J.D. Viscoelastic and morphological behavior of hybridstyryl-based polyhedral oligomeric silsesquioxane (POSS) copolymers. J. Polym. Sci. Part Polym. Phys. 1998, 36, 1857–1872. [Google Scholar] [CrossRef]
- Bizet, S.; Galy, J.; Gerard, J.F. Structure-Property Relationships in Organic-Inorganic Nanomaterials Based on Methacryl-POSS and Dimethacrylate Networks. Macromolecules 2006, 39, 2574–2583. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Franczyk, A.; Marciniec, B. Synthesis of monofunctionalized POSS through hydrosilylation. J. Organomet. Chem. 2018, 872, 73–78. [Google Scholar] [CrossRef]
- Saito, H. Process for Production of Powder of Cage Silsesquioxane Compound. US Patent 2010/0081837 A1, 2010. [Google Scholar]
- Odian, G. Principles of Polymerization, 4th ed.; Wiley-Interscience, John Wiley & Sons, Inc.: Hoboken, NY, USA, 2004. [Google Scholar]
- Andrzejewska, E.; Podgórska-Golubska, M.; Stępniak, I.; Andrzejewski, M. Photoinitiated polymerization in ionic liquids: Kinetics and viscosity effects. Polymer 2009, 50, 2040–2047. [Google Scholar] [CrossRef]
- Lekishvili, N.G.; Nadareishvili, N.I.; Khananashvili, Z.M.; Zaikov, G.E. Polymers for fiber optics. In Chemical and Physical Properties of Polymers; Zaikov, G.E., Kozlowski, R., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Jaso, V.; Stoiljkovic, D.; Radicevic, R.; Bera, O. Kinetic modeling of bulk free-radical polymerization of methyl methacrylate. Polym. J. 2013, 45, 631–636. [Google Scholar] [CrossRef]
- Matsumoto, A.; Tanaka, S.; Otsu, T. Synthesis and Characterization of Poly (l-adamantyl methacrylate): Effects of the Adamantyl Group on Radical Polymerization Kinetics and Thermal Properties of the Polymer. Macromolecules 1991, 24, 4017–4024. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Kim, G.-M.; Mather, P.T. Rheological Behavior of Entangled Polystyrene-Polyhedral Oligosilsesquioxane (POSS) Copolymers. Macromolecules 2007, 40, 544–554. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Bizet, S.; Galy, J.; Gerard, J.-F. Molecular dynamics simulation of organic-inorganic copolymers based on methacryl-POSS and methyl methacrylate. Polymer 2006, 47, 8219–8227. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Lee, H.-Y.; Shin, Y.-J.; Lee, D.-H. Preparation and properties of mma/1-propylmethacrylate-POSS copolymer with atom transfer radical polymerization. Chin. J. Polym. Sci. 2008, 26, 533–537. [Google Scholar] [CrossRef]
- Molina, D.; Levi, M.; Turri, S.; Penso, M. Self-Assembly of Methacrylic Nanostructured Copolymers Containing Polyhedral Oligomeric Silsesquioxanes. e-Polymers 2007, 7, 1–13. [Google Scholar] [CrossRef]
- Taherzadeh, H.; Ishida, Y.; Kameyama, A. Phase-separated structures of random methacrylate copolymers with pendant POSS moieties. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. [Google Scholar] [CrossRef]
- Cook, W.D.; Chausson, S.; Chen, F.; Le Pluart, L.; Bowman, C.N.; Scott, T.F. Photopolymerization kinetics, photorheology and photoplasticity of thiol–ene–allylic sulfide networks. Polym. Int. 2008, 57, 469–478. [Google Scholar] [CrossRef]
- Ligon, S.C.; Seidler, K.; Gorsche, C.; Griesser, M.; Moszner, N.; Liska, R. Allyl Sulfides and a-Substituted Acrylates as Addition–Fragmentation Chain Transfer Agents for Methacrylate Polymer Networks. J. Polym. Sci. Part Polym. Chem. 2016, 54, 394–406. [Google Scholar] [CrossRef]
- Gorsche, C.; Harikrishna, R.; Baudis, S.; Knaack, P.; Husar, B.; Laeuger, J.; Hoffmann, H.; Liska, R. Real Time-NIR/MIR-Photorheology: A Versatile Tool for the in Situ Characterization of Photopolymerization Reactions. Anal. Chem. 2017, 89, 4958–4968. [Google Scholar] [CrossRef]
- Buback, M.; Junkers, T. Termination Kinetics of tert-Butyl Methacrylate and of n-Butyl Methacrylate Free-Radical Bulk Homopolymerizations. Macromol. Chem. Phys. 2006, 207, 1640–1650. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M.; Davis, T.P.; Garcıa, N.; Gilbert, R.G.; Hutchinson, R.A.; Kajiwara, A.; Kamachi, M.; Lacık, I.; Russell, G.T. Critically Evaluated Rate Coefficients for Free-Radical Polymerization, 4, Propagation Rate Coefficients for Methacrylates with Cyclic Ester Groups. Macromol. Chem. Phys. 2003, 204, 1338–1350. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, S.; Cardoen, G.; Burgaz, E.; Gido, S.P.; Coughlin, E.B. Polymer Nanocomposites through Controlled Self-Assembly of Cubic Silsesquioxane Scaffolds. Macromolecules 2004, 37, 8606–8611. [Google Scholar] [CrossRef]
- Romo-Uribe, A. Viscoelasticity and microstructure of POSS-methyl methacrylate nanocomposites. Dynamics and entanglement dilution. Polymer 2018, 148, 27–38. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Mather, P.T. Vertex Group Effects in Entangled Polystyrene-Polyhedral Oligosilsesquioxane (POSS) Copolymers. Macromolecules 2009, 42, 1142–1152. [Google Scholar] [CrossRef]
- Amir, N.; Levina, A.; Silverstein, M.S. Nanocomposites Through Copolymerization of a Polyhedral Oligomeric Silsesquioxane and Methyl Methacrylate. J. Polym. Sci. Part Polym. Chem. 2007, 45, 4264–4275. [Google Scholar] [CrossRef]













| LM/1M-POSS | LM/TSM | ||
|---|---|---|---|
| 1M-POSS Concentration | TSM Concentration | ||
| mol % | wt % | mol % | wt % |
| 0.0 | 0.0 | 0.0 | 0.0 |
| 0.5 | 1.8 | 2.0 | 3.3 |
| 1.0 | 3.6 | 4.0 | 6.5 |
| 2.0 | 7.0 | 8.0 | 12.6 |
| 4.0 | 13.4 | 12.0 | 18.5 |
| 6.0 | 19.1 | 20.0 | 29.4 |
| 8.0 | 24.4 | 50.0 | 62.4 |
| 10.0 | 29.2 | 80.0 | 86.9 |
| 12.0 | 33.6 | 100.0 | 100.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowska, A.; Przadka, D.; Dudziec, B.; Szczesniak, K.; Andrzejewska, E. Anchor Effect in Polymerization Kinetics: Case of Monofunctionalized POSS. Polymers 2019, 11, 515. https://doi.org/10.3390/polym11030515
Marcinkowska A, Przadka D, Dudziec B, Szczesniak K, Andrzejewska E. Anchor Effect in Polymerization Kinetics: Case of Monofunctionalized POSS. Polymers. 2019; 11(3):515. https://doi.org/10.3390/polym11030515
Chicago/Turabian StyleMarcinkowska, Agnieszka, Dawid Przadka, Beata Dudziec, Katarzyna Szczesniak, and Ewa Andrzejewska. 2019. "Anchor Effect in Polymerization Kinetics: Case of Monofunctionalized POSS" Polymers 11, no. 3: 515. https://doi.org/10.3390/polym11030515