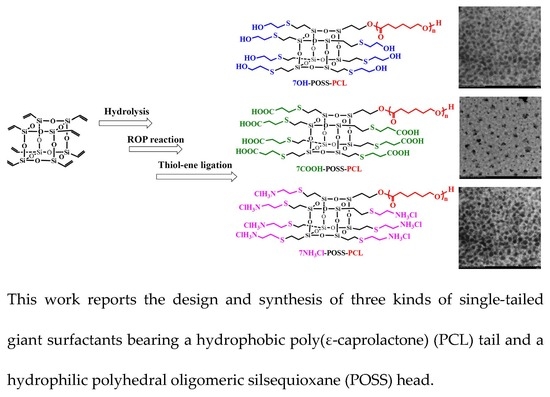
Versatile Construction of Single-Tailed Giant Surfactants with Hydrophobic Poly(ε-caprolactone) Tail and Hydrophilic POSS Head
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Monohydroxyl Heptavinyl Substituted POSS (VPOSS-OH)
2.3. Preparation of Poly(ε-caprolactone) End-Capped with Heptavinyl Substituted POSS (VPOSS-PCL)
2.4. Typical Procedure for Synthesizing Single-Tailed Giant Surfactants (7R-POSS-PCL)
2.5. Self-Assembly of Giant Surfactants in Aqueous Solution
2.6. Characterizations
3. Results and Discussion
3.1. Structure Characterization of Functional Initiator VPOSS-OH
3.2. Synthesis and Characterization of VPOSS-PCL
3.3. Synthesis and Characterization of Single-Tailed Giant Surfactants 7R-POSS-PCL
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Karsa, D.R. Industrial Applications of Surfactants IV; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 1–23. [Google Scholar]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional block copolymers: Nanostructured materials with emerging applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Lu, X.Y.; Wang, W.Y.; Kang, N.-G.; Mays, J.W. Block copolymers: Synthesis, self-Assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.S.; Liu, S.Y. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289–7325. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Reynhout, I.C.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Synthesis of polymer–biohybrids: From small to giant surfactants. Acc. Chem. Res. 2009, 42, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; Cheng, S.Z.D. Toward rational and modular molecular design in soft matter engineering. Chinese J. Polym. Sci. 2015, 33, 797–814. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Yu, X.F.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.F.; Li, Y.W.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular nanoparticles are unique elements for macromolecular science: From “nanoatoms” to giant molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Yu, X.F.; Li, Y.W.; Dong, X.-H.; Yue, K.; Lin, Z.W.; Feng, X.Y.; Huang, M.J.; Zhang, W.-B.; Cheng, S.Z.D. Giant surfactants based on molecular nanoparticles: Precise synthesis and solution self-assembly. J. Polym. Sci. Part B: Polym. Phys. 2014, 52, 1309–1325. [Google Scholar] [CrossRef]
- Dong, X.-H.; Hsu, C.-H.; Li, Y.W.; Liu, H.; Wang, J.; Huang, M.J.; Yue, K.; Sun, H.-J.; Wang, C.-L.; Yu, X.F.; et al. Supramolecular crystals and crystallization with nanosized motifs of giant molecules. In Polymer Crystallization I: From Chain Microstructure to Processing; Auriemma, F., Alfonso, G.C., de Rosa, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 276, pp. 183–213. [Google Scholar]
- Zhang, W.-B.; Wu, X.-L.; Yin, G.-Z.; Shao, Y.; Cheng, S.Z.D. From protein domains to molecular nanoparticles: What can giant molecules learn from proteins? Mater. Horiz. 2017, 4, 117–132. [Google Scholar]
- Tang, W.; Yue, K.; Cheng, S.Z.D. Molecular topology effects in self-assembly of giant surfactants. Acta Polym. Sin. 2018, 8, 959–972. [Google Scholar]
- Liu, Z.G.; Kong, D.Y.; Dong, X.-H. Two-dimensional assembly of giant molecules. Sci. China Chem. 2018, 61, 17–24. [Google Scholar] [CrossRef]
- Li, Q.X.; Wang, Z.; Yin, Y.H.; Jiang, R.; Li, B.H. Self-assembly of giant amphiphiles based on polymer-tethered nanoparticle in selective solvents. Macromolecules 2018, 51, 3050–3058. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cui, J.; Han, Y.Y.; Jiang, W. Effect of chain architecture on phase behavior of giant surfactant constructed from nanoparticle monotethered by single diblock copolymer chain. Langmuir 2019, 35, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Zhang, W.-B. The pursuit of precision in macromolecular science: Concepts, trends, and perspectives. Polymer 2018, 155, 235–247. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Blanco, I. The rediscovery of POSS: A molecule rather than a filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Sun, H.Y.; Gao, C. Superstructured assembly of nanocarbons: Fullerenes, nanotubes, and graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic–inorganic polyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Li, Y.W.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.J.; Liu, H.; Feng, X.Y.; Lin, Z.W.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Yue, K.; Liu, C.; Guo, K.; Wu, K.; Dong, X.-H.; Liu, H.; Huang, M.J.; Wesdemiotis, C.; Cheng, S.Z.D.; Zhang, W.-B. Exploring shape amphiphiles beyond giant surfactants: Molecular design and click synthesis. Polym. Chem. 2013, 4, 1056–1067. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.W.; Dong, X.-H.; Yu, X.F.; Guo, K.; Su, H.; Yue, K.; Wesdemiotis, C.; Cheng, S.Z.D.; Zhang, W.-B. Giant gemini surfactants based on polystyrene–hydrophilic polyhedral oligomeric silsesquioxane shape amphiphiles: Sequential “click” chemistry and solution self-assembly. Chem. Sci. 2013, 4, 1345–1352. [Google Scholar] [CrossRef]
- Su, H.; Zheng, J.; Wang, Z.; Lin, F.; Feng, X.Y.; Dong, X.-H.; Becker, M.L.; Cheng, S.Z.D.; Zhang, W.-B.; Li, Y.W. Sequential triple “click” approach toward polyhedral oligomeric silsesquioxane-based multiheaded and multitailed giant surfactants. ACS Macro Lett. 2013, 2, 645–650. [Google Scholar] [CrossRef]
- Yu, X.F.; Yue, K.; Hsieh, I.-F.; Li, Y.W.; Dong, X.-H.; Liu, C.; Xin, Y.; Wang, H.-F.; Shi, A.-C.; Newkome, G.R.; et al. Giant surfactants provide a versatile platform for sub-10-nm nanostructure engineering. Proc. Natl. Acad. Sci., USA 2013, 110, 10078–10083. [Google Scholar] [CrossRef]
- Wu, K.; Huang, M.J.; Yue, K.; Liu, C.; Lin, Z.W.; Liu, H.; Zhang, W.; Hsu, C.-H.; Shi, A.-C.; Zhang, W.-B.; et al. Asymmetric giant “bolaform-like” surfactants: Precise synthesis, phase diagram, and crystallization-induced phase separation. Macromolecules 2014, 47, 4622–4633. [Google Scholar] [CrossRef]
- Yue, K.; Huang, M.J.; Marson, R.L.; He, J.L.; Huang, J.; Zhou, Z.; Wang, J.; Liu, C.; Yan, X.S.; Wu, K.; et al. Geometry induced sequence of nanoscale Frank–Kasper and quasicrystal mesophases in giant surfactants. Proc. Natl. Acad. Sci. USA 2016, 113, 14195–14200. [Google Scholar] [CrossRef]
- Yue, K.; Liu, C.; Huang, M.J.; Huang, J.; Zhou, Z.; Wu, K.; Liu, H.; Lin, Z.W.; Shi, A.-C.; Zhang, W.-B.; et al. Self-assembled structures of giant surfactants exhibit a remarkable sensitivity on chemical compositions and topologies for tailoring sub-10 nm nanostructures. Macromolecules 2017, 50, 303–314. [Google Scholar] [CrossRef]
- Li, Z.X.; Fu, Y.; Li, Z.; Nan, N.; Zhu, Y.M.; Li, Y.W. Froth flotation giant surfactants. Polymer 2019, 162, 58–62. [Google Scholar] [CrossRef]
- Huang, M.J.; Yue, K.; Huang, J.; Liu, C.; Zhou, Z.; Wang, J.; Wu, K.; Shan, W.; Shi, A.-C.; Cheng, S.Z.D. Highly asymmetric phase behaviors of polyhedral oligomeric silsesquioxane-based multiheaded giant surfactants. ACS Nano 2018, 12, 1868–1877. [Google Scholar] [CrossRef]
- Wang, X.-M.; Shao, Y.; Xu, J.; Jin, X.; Shen, R.-H.; Jin, P.-F.; Shen, D.-W.; Wang, J.; Li, W.H.; He, J.L.; et al. Precision synthesis and distinct assembly of double-chain giant surfactant regioisomers. Macromolecules 2017, 50, 3943–3953. [Google Scholar] [CrossRef]
- Wang, X.-M.; Shao, Y.; Jin, P.-F.; Jiang, W.; Hu, W.; Yang, S.; Li, W.H.; He, J.L.; Ni, P.H.; Zhang, W.-B. Influence of regio-configuration on the phase diagrams of double-chain giant surfactants. Macromolecules 2018, 51, 1110–1119. [Google Scholar] [CrossRef]
- He, J.L.; Yue, K.; Liu, Y.Q.; Yu, X.F.; Ni, P.H.; Cavicchi, K.A.; Quirk, R.P.; Chen, E.Q.; Cheng, S.Z.D.; Zhang, W.-B. Fluorinated polyhedral oligomeric silsesquioxane-based shape amphiphiles: Molecular design, topological variation, and facile synthesis. Polym. Chem. 2012, 3, 2112–2120. [Google Scholar] [CrossRef]
- Feher, F.J.; Wyndham, K.D.; Baldwin, R.K.; Soulivong, D.; Ziller, J.W.; Lichtenhan, J.D. Methods for effecting monofunctionalization of (CH2=CH)8Si8O12. Chem. Commun. 1999, 14, 1289–1290. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; He, J.L.; Zhang, M.Z.; Ni, P.H. Aapplications of click chemistry in synthesis of tolological polymers. Acta Polym. Sin. 2013, 3, 300–319. [Google Scholar]










| Entry | Samples | Mn, NMRa | Mn, GPCb | Mw/Mnb |
|---|---|---|---|---|
| 1 | VPOSS-PCL-1 | 2300 | 3800 | 1.09 |
| 2 | VPOSS-PCL-2 | 4100 | 5000 | 1.07 |
| 3 | VPOSS-PCL-3 | 5400 | 7700 | 1.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Q.; Xu, J.; Zhang, M.; He, J.; Ni, P. Versatile Construction of Single-Tailed Giant Surfactants with Hydrophobic Poly(ε-caprolactone) Tail and Hydrophilic POSS Head. Polymers 2019, 11, 311. https://doi.org/10.3390/polym11020311
Qian Q, Xu J, Zhang M, He J, Ni P. Versatile Construction of Single-Tailed Giant Surfactants with Hydrophobic Poly(ε-caprolactone) Tail and Hydrophilic POSS Head. Polymers. 2019; 11(2):311. https://doi.org/10.3390/polym11020311
Chicago/Turabian StyleQian, Qiangyu, Jun Xu, Mingzu Zhang, Jinlin He, and Peihong Ni. 2019. "Versatile Construction of Single-Tailed Giant Surfactants with Hydrophobic Poly(ε-caprolactone) Tail and Hydrophilic POSS Head" Polymers 11, no. 2: 311. https://doi.org/10.3390/polym11020311