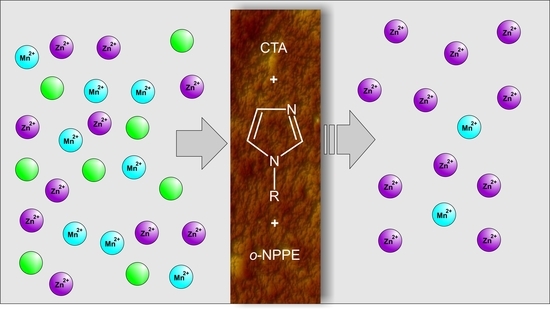
The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
2.2. Polymer Inclusion Membrane Preparation
2.3. Characteristics of PIMs
2.4. Transport Studies
3. Results and Discussion
3.1. The Characteristics of Membranes
3.2. Transport of Zn(II) and Mn(II) ions across PIMs
3.3. Recovery of Metal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lead and Zinc Statistics. Available online: http://www.ilzsg.org/static/statistics.aspx (4 December 2018).
- Jafari, H.; Abdollahi, H.; Gharabaghi, M.; Balesini, A.A. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. Sep. Purif. Technol. 2018, 197, 210–219. [Google Scholar] [CrossRef]
- Cole, P.M.; Sole, K.C. Zinc solvent extraction in the process industries. Miner. Process. Extr. Metall. Rev. 2003, 24, 91–137. [Google Scholar] [CrossRef]
- Gotfryd, L.; Chmielarz, A.; Szołomicki, Z. Recovery of zinc from arduous wastes using solvent extraction technique. Part II. Pilot plant tests. Physicochem. Probl. Miner. Process. 2011, 47, 249–258. [Google Scholar]
- Charewicz, W.; Holowiecka, B.A.; Walkowiak, W. Selective flotation of zinc(II) and silver(I) ions from dilute aqueous solutions. Sep. Sci. Technol. 1999, 34, 2447–2460. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Bartsch, R.A. Ion Flotation of Zinc(II) and Cadmium(II) with Proton-Ionizable Lariat Ethers—The effect of cavity size. Sep. Purif. Technol. 2006, 48, 264–269. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Brandt, K.; Porwolik-Czomperlik, I. Ion flotation of zinc(II) and cadmium(II) in the presence of side-armed diphosphaza-16-crown-6ethers. Sep. Sci. Technol. 2003, 38, 633–645. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Ulewicz, M.; Walkowiak, W.; Girek, T.; Jablonska, J. The effect of tautomeric rearrangement on the separation of Zn(II) and Cd(II) in ion flotation process with 4-thiazolidinone derivatives. Miner. Eng. 2002, 15, 677–682. [Google Scholar] [CrossRef]
- Kozlowski, C.; Ulewicz, M.; Walkowiak, W. Separation of zinc and cadmium ions from aqueous chloride solutions by ion flotation and liquid membranes. Physicochem. Probl. Miner. Process. 2000, 34, 141–151. [Google Scholar]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E-S.Z. Removal of zinc ions from aqueous solution using a cation exchange resin. Chem. Eng. Res. Des. 2013, 91, 165–173. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V. Recovery of zinc from aqueous solutions by ion exchange process—A review. J. Metall. Mater. Sci. 2005, 47, 119–128. [Google Scholar]
- Sobianowska-Turek, A.; Ulewicz, M.; Sobianowska, K. Ion flotation and solvent sublation of zinc(II) and manganese(II) in the presence of proton-ionizable lariat ethers. Physicochem. Probl. Miner. Process. 2016, 52, 1048–1060. [Google Scholar] [CrossRef]
- Cheng, Q.; Dong, H. Solvent Sublation Using Dithizone as a Ligand for Determination of Trace Elements in Water Samples. Microchim. Acta 2005, 150, 59–65. [Google Scholar] [CrossRef]
- Dabek, L. Sorption of zinc ions from aqueous solutions on regenerated activated carbons. J. Hazard. Mater. 2003, 101, 191–201. [Google Scholar] [CrossRef]
- Kononova, O.N.; Kuzetsova, M.A.; Melnikov, A.M.; Karplyakova, N.S.; Kononov, Y.S. Sorption recovery of copper(II) and zinc(II) from aqueous chloride solutions. J. Serb. Chem. Soc. 2014, 79, 1037–1049. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Amado, F.D.R.; Bischoff, M.R.; Ferreira, C.A.; Bernardes, A.M.; Ferreira, J.Z. Transport of zinc complexes through an anion exchange membrane. Desalination 2008, 227, 241–252. [Google Scholar] [CrossRef]
- Gasser, M.S.; El-Hefny, N.E.; Rizk, S.E.; Daoud, J.A. Transfer and Separation of Zn(II)/Co(II) by Supported Liquid Membrane Containing CYANEX 925 in Kerosene as Carrier. J. Phys. Sci. 2013, 24, 63–81. [Google Scholar]
- Ulewicz, M.; Kozłowski, C.; Walkowiak, W. Removal of Zn(II), Cd(II) and Cu(II) ions by polymer inclusion membrane with side-armed diphosphaza-16-crown-6-ethers. Physicochem. Probl. Miner. Process 2004, 38, 131–138. [Google Scholar]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of 1-alkylimidzoles for selective separation of zinc ions in the transport process across a polymeric inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Kosciuszko, A.; Gierszewska, M.; Ziuziakowski, K. The influence of the morphology and mechanical properties of polymer inclusion membranes (PIMs) on zinc ion separation from aqueous solutions. Polymers 2018, 10, 134. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kozłowski, C.A.; Koziol, J.J. Transport of Zn(II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers. Sep. Purif. Technol. 2007, 57, 430–434. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Nowicki, M.; Wisniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 132, 42319–42329. [Google Scholar] [CrossRef]
- Ulewicz, M.; Sadowska, K.; Biernat, J.F. Selective transport of Pb(II) across polymer inclusion membrane using imidazole azocrown ethers as carriers. Physicochem. Probl. Miner. Process. 2007, 41, 133–143. [Google Scholar]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130. [Google Scholar]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer and supported membranes with 1-decyl-4-methylimidazole for pertraction of transition metal ions. Sep. Sci. Technol. 2014, 49, 1713–1721. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membrane (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. The application of membrane extraction in the separation of zinc and cadmium ions. Desalin. Water Treat. 2018, 128, 140–147. [Google Scholar] [CrossRef]
- Reddy, T.B. Linden’s Handbook of Batteries, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2011; ISBN 9780071624213. [Google Scholar]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Bernades, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Veloso, L.R.S.; Rodrigues, L.E.O.C.; Ferreira, D.A.; Magalhäes, F.S.; Mensur, M.B. Development of a hydrometallurgical route for the recovery of zinc and manganese from spent alkaline batteries. J. Power Sources 2005, 152, 295–302. [Google Scholar] [CrossRef]
- Bartsch, R.A.; Way, J.D. Chemical Separation with Liquid Membranes; ASC: Washington, DC, USA, 1996; ISBN 9780841234475. [Google Scholar]
- Bond, A.H.; Dietz, M.L.; Rogers, R.D. Metal-Ion Separation and Preconcentration; Progress and Opportunities; ACS: Washington, DC, USA, 1999; ISBN 0-8412-3594-5. [Google Scholar]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 44–451. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Proc. Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective transport of Cu(II) across a polymer inclusion membrane with 1-alkylimidazole from nitrate solutions. Sep. Sci. Technol 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Inês, M.; Almeida, G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci 2012, 415—416, 9–23. [Google Scholar] [CrossRef]
- Pernak, J.; Krysiski, J.; Skrzypczak, A. Bakterizide wirkung von iminiumverbindungen. Tenside Surfactants Deterg. 1987, 24, 276–286. [Google Scholar]
- Lenarcik, B.; Ojczenasz, P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid—Base properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Wolf, J.R.; Strieder, W. Surface and void tortuosities for a random fiber bed: Overlapping, parallel cylinders of several radii. J. Membr. Sci. 1990, 49, 103–115. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymer plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mater. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; San Miguel, E.R.; Muhammed, M.; Gyves, J. Transport characterization of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celikas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) thought polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Benosmane, N.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Selective transport of metal ions across polymer inclusion membranes containing calix[4]resorcinares. Sep. Purif. Technol. 2009, 65, 211–219. [Google Scholar] [CrossRef]
- Resina, M.; Macanas, J.; de Gyvesb, J.; Munoz, M. Development and characterization of hybrid membranes based on an organic matrix modified with silanes for metal separation. J. Membr. Sci. 2007, 289, 150–158. [Google Scholar] [CrossRef]










| Carrier in the CTA-o-NPPE Membrane | Effective Pore Size, µm | Tortuosity | Roughness (Rq), nm |
|---|---|---|---|
| 1-hexyl-imidazole (1) | 0.050 ± 0.002 | 2.32 | 5.90 ± 0.05 |
| 1-heptyl-imidazole (2) | 0.053 ± 0.002 | 2.35 | 6.10 ± 0.05 |
| 1-octyl-imidazole (3) | 0.054 ± 0.002 | 2.38 | 6.50 ± 0.05 |
| 1-nonyl-imidazole (4) | 0.055 ± 0.002 | 2.64 | 6.70 ± 0.05 |
| 1-decyl-midazole (5) | 0.057 ± 0.002 | 2.81 | 7.20 ± 0.05 |
| Carrier | Metal Ions | J, μmol/m2⋅s | SZn(II)/Mn(II) |
|---|---|---|---|
| 1 | Zn(II) | 1.97 | Zn(II) > Mn(II) 19.7 |
| Mn(II) | 0.10 | ||
| 2 | Zn(II) | 2.04 | Zn(II) > Mn(II) 15.7 |
| Mn(II) | 0.13 | ||
| 3 | Zn(II) | 2.13 | Zn(II) > Mn(II) 14.2 |
| Mn(II) | 0.15 | ||
| 4 | Zn(II) | 2.43 | Zn(II) > Mn(II) 12.8 |
| Mn(II) | 0.19 | ||
| 5 | Zn(II) | 2.65 | Zn(II) > Mn(II) 11.0 |
| Mn(II) | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzyminska-Lenarcik, E.; Ulewicz, M. The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers 2019, 11, 242. https://doi.org/10.3390/polym11020242
Radzyminska-Lenarcik E, Ulewicz M. The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers. 2019; 11(2):242. https://doi.org/10.3390/polym11020242
Chicago/Turabian StyleRadzyminska-Lenarcik, Elzbieta, and Malgorzata Ulewicz. 2019. "The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions" Polymers 11, no. 2: 242. https://doi.org/10.3390/polym11020242