Effect of Flank Rotation on the Photovoltaic Properties of Dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-Based Narrow Band Gap Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Monomers and Copolymer
2.1.1. 2-iodo-4,5-didecylthiophene (T-I)
2.1.2. 2-trimethylsilylethynyl-4,5-didecylthiophene (TE-Si)
2.1.3. 2-(4,5-didecylthien-2-yl)acetylene (TE)
2.1.4. 5,10-di(4,5-didecylthien-2-yl-ethynyl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene (DTBDT-TE)
2.1.5. 2,7-di(trimethylstannyl)-5,10-di(4,5-didecylthien-2-yl-ethynyl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene (DTBDT-TESn)
2.1.6. Synthesis of PDTBDT-TE-DTNT
3. Results and Discussion
3.1. Synthesis and Characterization of the Monomers and Copolymers
3.2. Absorption and Aggregation Characteristics of the Copolymers
3.2.1. Absorption Spectra of the Copolymer
3.2.2. Aggregation of the Copolymers in Solution and Solid State
3.3. Electrochemical Characteristic of the Copolymers
3.4. Hole Mobilities of the Blend Films from the Copolymers
3.5. Photovoltaic Characteristics and Optical Modeling of the Device from the Copolymers
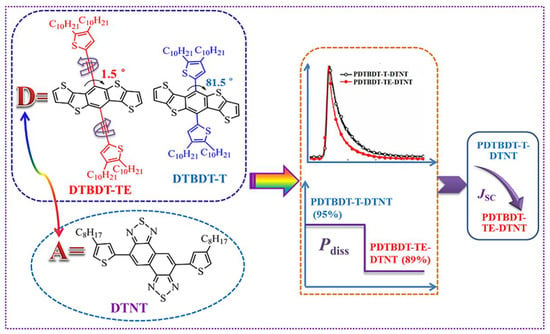
3.6. Time-Resolved Photoluminescence of the Copolymers
3.7. Computational Consideration of the Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.Q.; Dante, M.; Heeger, A.J. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, J.A.; Beiley, Z.M.; Hoke, E.T.; Mateker, W.R.; Douglas, J.D.; Collins, B.A.; Tumbleston, J.R.; Graham, K.R.; Amassian, A.; Ade, H.; et al. The importance of fullerene percolation in the mixed regions of polymer-fullerene bulk heterojunction solar cells. Adv. Energy Mater. 2013, 3, 364–374. [Google Scholar] [CrossRef]
- Huo, L.; Hou, J.; Zhang, S.; Chen, H.-Y.; Yang, Y. A polybenzo[1,2-b:4,5-b′]dithiophene derivative with deep HOMO level and its application in high-performance polymer solar cells. Angew. Chem. Int. Edit. 2010, 122, 1542–1545. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Price, S.C.; Knight, K.J.; You, W. Enhanced photovoltaic performance of low-bandgap polymers with deep LUMO levels. Angew. Chem. Int. Edit. 2010, 122, 8164–8167. [Google Scholar] [CrossRef]
- Kan, B.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Yang, X.; Zhang, M.; Zhang, H.; Russell, T.P.; et al. Small molecules based on alkyl/alkylthio-thieno[3,2-b]thiophene substituted benzo[1,2-b:4,5-b′]dithiophene for solution-processed solar cells with high performance. Chem. Mater. 2015, 27, 8414–8423. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y. Side-chain engineering of high-efficiency conjugated polymer photovoltaic materials. Sci. China Chem. 2015, 58, 192–209. [Google Scholar] [CrossRef]
- Wang, X.; Du, Z.; Dou, K.; Jiang, H.; Gao, C.; Han, L.; Yang, R. A maverick asymmetrical backbone with distinct flanked twist angles modulating the molecular aggregation and crystallinity for high performance nonfullerene solar cells. Adv. Energy Mater. 2018, 1802530. [Google Scholar] [CrossRef]
- Chen, W.; Huang, G.; Li, X.; Wang, H.; Li, Y.; Jiang, H.; Zheng, N.; Yang, R. Side-chain-promoted benzodithiophene-based conjugated polymers toward striking enhancement of photovoltaic properties for polymer solar cells. ACS Appl. Mater. Interfaces 2018, 10, 42747–42755. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Hou, J. Benzo[1,2-b:4,5-b′]dithiophene-based conjugated polymers: Band gap and energy level control and their application in polymer solar cells. Polym. Chem. 2011, 2, 2453–2461. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Huo, L.; Zhang, M.; Hou, J. Molecular design toward highly efficient photovoltaic polymers based on two-dimensional conjugated benzodithiophene. Acc. Chem. Res. 2014, 47, 1595–1603. [Google Scholar] [CrossRef]
- Cai, Y.; Huo, L.; Sun, Y. Recent advances in wide-bandgap photovoltaic polymers. Adv. Mater. 2017, 29, 1605437. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, H.; Huang, G.; Zhang, J.; Cai, M.; Wan, X.; Yang, R. High-efficiency ternary polymer solar cells based on intense FRET energy transfer process. Sol. RRL 2018, 2, 1800101. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, S.; Guo, X.; Xu, F.; Li, Y.; Hou, J. Replacing alkoxy groups with alkylthienyl groups: A feasible approach to improve the properties of photovoltaic polymers. Angew. Chem. Int. Ed. 2011, 50, 9697–9702. [Google Scholar] [CrossRef]
- Duan, R.; Ye, L.; Guo, X.; Huang, Y.; Wang, P.; Zhang, S.; Zhang, J.; Huo, L.; Hou, J. Application of two-dimensional conjugated benzo[1,2-b:4,5-b′]dithiophene in quinoxaline-based photovoltaic polymers. Macromolecules 2012, 45, 3032–3038. [Google Scholar] [CrossRef]
- Qian, D.; Ye, L.; Zhang, M.; Liang, Y.; Li, L.; Huang, Y.; Guo, X.; Zhang, S.; Tan, Z.; Hou, J. Design, application, and morphology study of a new photovoltaic polymer with strong aggregation in solution state. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, J.Y.; Bang, S.-M.; Lim, B.; Lee, J.; Na, S.-I. A feasible random copolymer approach for high efficiency polymeric photovoltaic cells. J. Mater. Chem. A 2016, 4, 11439–11445. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Zhang, W.; Meng, X.; Zou, X.; Rasi, D.D.C.; Ma, W.; Yartsev, A.; Andersson, M.R.; Janssen, R.A.J.; et al. 8.0% Efficient all-polymer solar cells with high photovoltage of 1.1 V and internal quantum efficiency near unity. Adv. Energy Mater. 2018, 8, 1700908. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Zhao, W.; Liu, D.; Yao, H.; Hou, J. Side chain selection for designing highly efficient photovoltaic polymers with 2D-Conjugated structure. Macromolecules 2014, 47, 4653–4659. [Google Scholar] [CrossRef]
- Jiang, J.-M.; Lin, H.-K.; Lin, Y.-C.; Chen, H.-C.; Lan, S.-C.; Chang, C.-K.; Wei, K.-H. Side chain structure affects the photovoltaic performance of two-dimensional conjugated polymers. Macromolecules 2014, 47, 70–78. [Google Scholar] [CrossRef]
- Kim, J.-H.; Song, C.E.; Kim, B.; Kang, I.-N.; Shin, W.S. Thieno[3,2-b]thiophene-substituted benzo[1,2-b:4,5-b′]dithiophene as a promising building block for low bandgap semiconducting polymers for high-performance single and tandem organic photovoltaic cells. Chem. Mater. 2014, 26, 1234–1242. [Google Scholar] [CrossRef]
- Son, H.J.; Lu, L.; Chen, W.; Xu, T.; Zheng, T.; Carsten, B.; Strzalka, J.; Darling, S.B.; Chen, L.X.; Yu, L. Synthesis and photovoltaic effect in dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene- based conjugated polymers. Adv. Mater. 2013, 25, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.; Ma, W.; Huang, Y.; Huo, L.; Guo, X.; Zhang, M.; Ade, H.; Hou, J. PDT-S-T: A new polymer with optimized molecular conformation for controlled aggregation and π-π stacking and its application in efficient photovoltaic devices. Adv. Mater. 2013, 25, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, P.; Li, J.; Li, Y.; Wang, J.; Zhang, S.; Xia, Y.; Meng, X.; Fan, D.; Chu, J. Synthetically controlling the optoelectronic properties of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b: 4,5-b′]dithiophene-alt-diketopyrrolopyrrole conjugated polymers for efficient solar cells. J. Mater. Chem. A 2014, 2, 15316–15325. [Google Scholar] [CrossRef]
- Huo, L.; Liu, T.; Sun, X.; Cai, Y.; Heeger, A.J.; Sun, Y. Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%. Adv. Mater. 2015, 27, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Xia, Y.; Huang, F.; Luo, G.; Li, J.; Zhang, P.; Zhu, Y.; Yang, C.; Wu, H.; Cao, Y. An alkylthieno-2-yl flanked dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-based low band gap conjugated polymer for high performance photovoltaic solar cells. RSC Adv. 2015, 5, 12879–12885. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, K.; Jiang, X.-F.; Ying, L.; Huang, F.; Cao, Y. High-performance nonfullerene polymer solar cells based on imide-functionalized wide-bandgap polymers. Adv. Mater. 2017, 29, 1606396. [Google Scholar] [CrossRef]
- Guo, P.; Sun, J.; Sun, S.; Li, J.; Tong, J.; Zhao, C.; Zhu, L.; Zhang, P.; Yang, C.; Xia, Y. Effect of alkylthiophene spacers and fluorine on the optoelectronic properties of 5,10-bis(dialkyl- thien-2-yl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-alt–benzothiadiazole derivative copolymers. RSC Adv. 2017, 7, 22845–22854. [Google Scholar] [CrossRef]
- Gao, P.; Tong, J.; Guo, P.; Li, J.; Wang, N.; Li, C.; Ma, X.; Zhang, P.; Wang, C.; Xia, Y. Medium band gap conjugated polymers from acene derivatives and pentacyclic aromatic lactam as promising alternatives of poly(3-hexylthiophene) in photovoltaic application. J. Polym. Sci. Polym. Chem. 2018, 56, 85–95. [Google Scholar] [CrossRef]
- Huang, F.; Wu, H.; Wang, D.; Yang, W.; Cao, Y. Novel electroluminescent conjugated polyelectrolytes based on polyfluorene. Chem. Mater. 2004, 16, 708–716. [Google Scholar] [CrossRef]
- Xia, Y.; Su, X.; He, Z.; Ren, X.; Wu, H.; Cao, Y.; Fan, D. An alternating copolymer derived from indolo[3,2-b]carbazole and 4,7-di(thieno[3,2-b]thien-2-yl)-2,1,3-benzothiadiazole for photovoltaic cells. Macromol. Rapid Commun. 2010, 31, 1287–1292. [Google Scholar] [CrossRef]
- An, M.; Xie, F.; Geng, X.; Zhang, J.; Jiang, J.; Lei, Z.; He, D.; Xiao, Z.; Ding, L. A high-performance D-A copolymer based on dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one unit compatible with fullerene and nonfullerene acceptors in solar cells. Adv. Energy Mater. 2017, 7, 1602509. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A low-bandgap poly(2,7-carbazole) derivative for use in high-performance solar cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Zhong, W.; Xiao, J.; Sun, S.; Jiang, X.-F.; Lan, L.; Ying, L.; Yang, W.; Yip, H.-L.; Huang, F.; Cao, Y. Wide bandgap dithienobenzodithiophene-based π-conjugated polymers consisting of fluorinated benzotriazole and benzothiadiazole for polymer solar cells. J. Mater. Chem. C 2016, 4, 4719–4727. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Nie, W.; Tsai, H.; Yen, H.-J.; Mohite, A.D.; Gupta, G.; Dattelbaum, A.M.; William, D.J.; Cha, K.C.; Yang, Y.; et al. Structural design of benzo[1,2-b:4,5-b′]dithiophene-based 2D conjugated polymers with bithienyl and terthienyl substituents toward photovoltaic applications. Macromolecules 2014, 47, 1008–1020. [Google Scholar] [CrossRef]
- Zhu, D.; Bao, X.; Zhu, Q.; Gu, C.; Qiu, M.; Wen, S.; Wang, J.; Shahid, B.; Yang, R. Thienothiophene-based copolymers for high-performance solar cells, employing different orientations of the thiazole group as a π bridge. Energy Environ. Sci. 2017, 10, 614–620. [Google Scholar] [CrossRef]
- Tong, J.; Li, J.; Zhang, P.; Ma, X.; Wang, M.; An, L.; Sun, J.; Guo, P.; Yang, C.; Xia, Y. Naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-based conjugated polymers consisting of oligothio- phenes for efficient polymer solar cells. Polymer 2017, 121, 183–195. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Gao, J.; Wang, D.; Yu, G.; Heeger, A.J. Electrochemical properties of luminescent polymers and polymer light-emitting electrochemical cells. Synth. Met. 1999, 99, 243–248. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, H.; Yang, C.; Li, Y. Synthesis and electroluminescence of novel copolymers containing crown ether spacers. J. Mater. Chem. 2003, 13, 800–806. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk-heterojunction solar cells-towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; An, L.; Lv, J.; Guo, P.; Wang, X.; Yang, C.; Xia, Y. Enhanced photovoltaic performance in D-π-A copolymers containing triisopropylsilylethynyl-substituted dithienobenzodithio- phene by modulating the electron-deficient units. Polymers 2019, 11, 12. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, D.H.; Luo, C.; Cao, Y.; Nguyen, T.-Q.; Bazan, G.C.; Heeger, A.J. Effects of solvent additives on morphology, charge generation, transport, and recombination in solution-processed small-molecule solar cells. Adv. Energy Mater. 2014, 4, 1301469. [Google Scholar] [CrossRef]
- Chu, T.-Y.; Song, O.-K. Hole mobility of N,N′-bis(naphthalen-1-yl)-N,N′-bis(phenyl) benzidine investigated by using space-charge-limited currents. Appl. Phys. Lett. 2007, 90, 203512. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupr´e, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–303. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Gevaerts, V.S.; Nuzzo, D.D.; Turbiez, M.; Mathijssen, S.G.J.; de Leeuw, D.M.; Wienk, M.M.; Janssen, R.A.J. Efficient solar cells based on an easily accessible diketopyrrolopyrrole polymer. Adv. Mater. 2010, 22, E242–E246. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.; Janssen, R.A.J. Nanoscale morphology of high-performance polymer solar cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef]
- Huo, L.; Ye, L.; Wu, Y.; Li, Z.; Guo, X.; Zhang, M.; Zhang, S.; Hou, J. Conjugated and nonconjugated substitution effect on photovoltaic properties of benzodifuran-based photovoltaic polymers. Macromolecules 2012, 45, 6923–6929. [Google Scholar] [CrossRef]
- Guo, X.; Cui, C.; Zhang, M.; Huo, L.; Huang, Y.; Hou, J.; Li, Y. High efficiency polymer solar cells based on poly(3-hexylthiophene)/indene-C70 bisadduct with solvent additive. Energy Environ. Sci. 2012, 5, 7943–7949. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Yao, Y.; Yang, Y. Investigation of annealing effects and film thickness dependence of polymer solar cells based on poly3-hexylthiophene. J. Appl. Phys. 2005, 98, 043704. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Wang, Y.; Li, H.; Tong, J.; Bao, X.; Xia, Y. Enhanced efficiency of polymer solar cells through synergistic optimization of mobility and tuning donor alloys by adding high-mobility conjugated polymers. J. Mater. Chem. C 2018, 6, 11015–11022. [Google Scholar] [CrossRef]
- Kan, B.; Feng, H.; Wan, X.; Liu, F.; Ke, X.; Wang, Y.; Wang, Y.; Zhang, H.; Li, C.; Hou, J.; et al. Small-molecule acceptor based on the heptacyclic benzodi(cyclopentadithiophene) unit for highly efficient nonfullerene organic solar cells. J. Am. Chem. Soc. 2017, 139, 4929–4934. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, L.; Hays, A.; Heeger, A.J.; Wang, G.; Bowers, J.E. Time-resolved photolumine- scence from poly[2-methoxy, 5-(2′-ethyl-hexyloxy)-p-phenylene-vinylene]: Solutions, gels, films, and blends. J. Chem. Phys. 1993, 98. [Google Scholar] [CrossRef]
- Wu, J.-L.; Chen, F.-C.; Hsiao, Y.S.; Chien, C.-F.; Chen, P.; Kuo, C.-H.; Huang, M.-H.; Hsu, C.-S. Surface plasmonic effects of metallic nanoparticles on the performance of polymer bulk heterojunction solar cells. ACS Nano 2011, 5, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yang, S.I.; Prathapan, S.; Miller, M.A.; Seth, J.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Synthesis and excited-state photodynamics in perylene-porphyrin dyads 2. Effects of porphyrin metalation state on the energy-transfer, charge-transfer, and deactivation channels. J. Phys. Chem. B 2001, 105, 8249–8258. [Google Scholar] [CrossRef]










| Copolymers | (nm) | (nm) | (nm) | (nm) | (ev) | HOMO (eV) 2 | LUMO (eV) 3 | |
|---|---|---|---|---|---|---|---|---|
| PDTBDT-TE-DTNT | 423, 545, 643, 699 | 789 | 426,549, 649,706 | 793 | 1.56 | 0.68 | –5.37 | –3.81 |
| PDTBDT-T-DTNT | 347, 484, 673, 726 | 809 | 350,491, 677,730 | 822 | 1.51 | 0.76 | –5.45 | –3.94 |
| Active Layer | Additives (DIO) | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) | µh (cm2·V−1·s−1) | |
|---|---|---|---|---|---|---|---|
| PDTBDT-TE-DTNT:PC71BM (W:W = 1:1) | 3% | 0.60 | 10.15 (10.01) a | 65.16 | 3.97 | 1.85 × 10−4 | 85 |
| PDTBDT-T-DTNT:PC71BM (W:W = 1:1) | 3% | 0.70 | 16.09 (15.98) a | 67.19 | 7.57 | 1.55 × 10−4 | 91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhu, L.; Guo, P.; Wang, X.; Tong, J.; Zhang, X.; Jia, Y.; Yang, R.; Xia, Y.; Wang, C. Effect of Flank Rotation on the Photovoltaic Properties of Dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-Based Narrow Band Gap Copolymers. Polymers 2019, 11, 239. https://doi.org/10.3390/polym11020239
Zhang M, Zhu L, Guo P, Wang X, Tong J, Zhang X, Jia Y, Yang R, Xia Y, Wang C. Effect of Flank Rotation on the Photovoltaic Properties of Dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-Based Narrow Band Gap Copolymers. Polymers. 2019; 11(2):239. https://doi.org/10.3390/polym11020239
Chicago/Turabian StyleZhang, Mingjing, Liangjian Zhu, Pengzhi Guo, Xunchang Wang, Junfeng Tong, Xiaofang Zhang, Yongjian Jia, Renqiang Yang, Yangjun Xia, and Chenglong Wang. 2019. "Effect of Flank Rotation on the Photovoltaic Properties of Dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-Based Narrow Band Gap Copolymers" Polymers 11, no. 2: 239. https://doi.org/10.3390/polym11020239