Chiral MnIII (Salen) Immobilized on Organic Polymer/Inorganic Zirconium Hydrogen Phosphate Functionalized with 3-Aminopropyltrimethoxysilane as an Efficient and Recyclable Catalyst for Enantioselective Epoxidation of Styrene
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
3. Preparation of Catalysts
3.1. Synthesis of Organic Polymer/Inorganic Zirconium Hydrogen Phosphate (ZSPP, ZPS-IPPA and ZPS-PVPA)
3.2. Synthesis of Organic Polymer/Inorganic Zirconium Hydrogen Phosphate Functionalized with 3-Aminopropyltrimethoxysilane
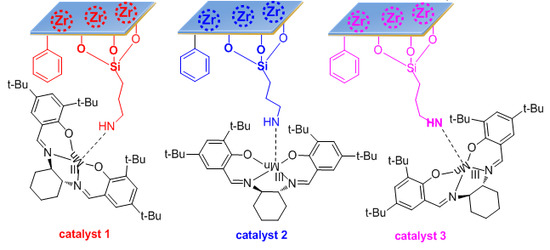
3.3. Immobilization of Chiral MnIII (Salen) Complex on the ZASPP, ZAPS-IPPA, and ZAPS-PVPA
3.4. Asymmetric Epoxidation
4. Results and Discussion
4.1. FT-IR Spectroscopy
4.2. DR UV–Vis Spectroscopy
4.3. XRD Analysis
4.4. Microscopic Analysis
4.5. X-ray Photoelectron Spectroscopy
4.6. Enantioselective Epoxidation of Styrene
4.7. Effect of Reaction Temperature
4.8. The Recycling of the Supported Chiral MnIII (Salen) Catalyst
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kamata, K.; Yonehara, K.; Sumida, Y.; Yamaguchi, K.; Hikichi, S.; Mizuno, N. Efficient epoxidation of olefins with >/=99% selectivity and use of hydrogen peroxide. Science 2003, 300, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Su, F.; Guo, W.; Zhang, S.; Guo, Y.; Hu, J. Epoxidation of styrene catalyzed by mesoporous propylthiol group-functionalized silica supported manganese(III) salen complexes with different pore morphologies. Microporous Mesoporous Mater. 2013, 169, 16–24. [Google Scholar] [CrossRef]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Zhang, W.; Jacobsen, E.N. Asymmetric olefin epoxidation with sodium hypochlorite catalyzed by easily prepared chiral manganese(III) salen complexes. J. Org. Chem. 1991, 56, 2296–2298. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, Y.; Xin, Q.; Li, C. Enantioselective epoxidation of olefins catalyzed by Mn (salen)/MCM-41 synthesized with a new anchoring method. Chem. Commun. 2002, 34, 2696–2697. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Ahmad, I.; Khan, N.H.; Abdi, S.H.R.; Pathak, K.; Jasra, R.V. Chiral MnIII salen complexes covalently bonded on modified MCM-41 and SBA-15 as efficient catalysts for enantioselective epoxidation of nonfunctionalized alkenes. J. Catal. 2006, 238, 134–141. [Google Scholar] [CrossRef]
- Reger, T.S.; Janda, K.D. Polymer-supported (salen)Mn catalysts for asymmetric epoxidation: A comparison between soluble and insoluble matrices. J. Am. Chem. Soc. 2000, 31, 6929–6934. [Google Scholar] [CrossRef]
- Smith, K.; Liu, C.H. Asymmetric epoxidation using a singly-bound supported Katsuki-type (salen) Mn complex. Chem. Commun. 2002, 8, 886–887. [Google Scholar] [CrossRef]
- Peng, M.; Chen, Y.J.; Tan, R.; Zheng, W.G. A highly efficient and recyclable catalyst—Dendrimer supported chiral salen MnIII complexes for asymmetric epoxidation. RSC Adv. 2013, 3, 20684–20692. [Google Scholar] [CrossRef]
- Liu, Y.Q.; An, Z.; Zhao, L.W.; Liu, H.; He, J. Enhanced catalytic efficiency in the epoxidation of alkenes for manganese complex encapsulated in the hydrophobic interlayer region of layered double hydroxides. Ind. Eng. Chem. Res. 2013, 52, 17821–17828. [Google Scholar] [CrossRef]
- He, S.; An, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide-based catalysts: Nanostructure design and catalytic performance. Chem. Commun. 2013, 49, 5912–5920. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.G.; Tan, R.; Yin, S.F.; Zhang, Y.Y.; Zhao, G.W.; Chen, Y.J.; Yin, D.H. Ionic liquid-functionalized graphene oxide as an efficient support for the chiral salen MnIII complex in asymmetric epoxidation of unfunctionalized olefins. Catal. Sci. Technol. Catal. Sci. Technol. 2015, 5, 2092–2102. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Allahresani, A.; Raissi, H. Grafting of a chiral MnIII complex on graphene oxide nanosheets and its catalytic activity for alkene epoxidation. RSC Adv. 2014, 4, 26087–26093. [Google Scholar] [CrossRef]
- Giuseppe, T.S.; Salvatrice, M.; Andrea, P.; Rosa, M.T.; Francesco, P.B.; Gaetano, A.T.; Antonino, G. Olefin epoxidation by a (salen) MnIII catalyst covalently grafted on glass beads. Catal. Sci. Technol. 2015, 5, 673–679. [Google Scholar]
- Ren, W.S.; Fu, X.K.; Bao, H.B.; Bai, R.F.; Ding, P.P.; Sui, B.L. Enantioselective epoxidation of unfunctionalized olefins catalyzed by chiral salen MnIII catalyst immobilized on zirconium oligostyrenylphosphonate-phosphate. Catal. Commun. 2009, 10, 788–793. [Google Scholar] [CrossRef]
- Zou, X.C.; Chen, S.C.; Ren, Y.R.; Shi, K.Y.; Li, J.; Fu, X.K. Recoverable MnIII (salen) supported on diamine modified zirconium poly(styrene-isopropenyl phosphonate)-phosphate as an efficient catalyst for epoxidation of unfunctionalized olefins. Sci. China Chem. 2012, 55, 2396–2406. [Google Scholar] [CrossRef]
- Gong, B.W.; Fu, X.K.; Chen, J.X.; Li, Y.D.; Zou, X.C.; Tu, X.B.; Ma, L.P. Synthesis of a new type of immobilized chiral salen MnIII complex as effective catalysts for asymmetric epoxidation of unfunctionalized olefins. J. Catal. 2009, 262, 9–17. [Google Scholar] [CrossRef]
- Zou, X.C.; Fu, X.K.; Li, Y.D.; Tu, X.B.; Fu, S.D.; Luo, Y.F.; Wu, X.J. Highly enantioselective epoxidation of unfunctionalized olefins catalyzed by chiral Jacobsen’s catalyst immobilized on phenoxyl modified Zirconium poly(styrene-phenylvinylphosphonate)-phosphate. Adv. Synth. Catal. 2010, 352, 163–170. [Google Scholar] [CrossRef]
- Zou, X.C.; Shi, K.Y.; Wang, C. Chiral MnIII (Salen) supported on tunable phenoxyl group modified zirconium poly(styrene-phenylvinylphosphonate)-phosphate as an efficient catalyst for epoxidation of unfunctionalized olefins. Chin. J. Catal. 2014, 35, 1446–1455. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Tan, R.; Zhao, G.W.; Luo, X.F.; Yin, D.H. Asymmetric epoxidation of unfunctionalized olefins accelerated by thermoresponsive self-assemblies in aqueous systems. Catal. Sci. Technol. 2016, 6, 488–496. [Google Scholar] [CrossRef]
- Li, Y.D.; Fu, X.K.; Gong, B.W.; Zou, X.C.; Tu, X.B.; Chen, J.X. Synthesis of novel immobilized tridentate Schiff base dioxomolybdenum(VI) complexes as efficient and reusable catalysts for epoxidation of unfunctionalized olefins. J. Mol. Catal. A Chem. 2010, 322, 55–62. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Khan, N.H.; Abdi, S.H.R.; Patel, S.T.; Lyer, P.K.; Jasra, R.V. A Highly Potential Analogue of Jacobsen Catalyst with In-built Phase Transfer Capability in Enantioselective Epoxidation of Nonfunctionalized Alkenes. J. Catal. 2002, 209, 99–104. [Google Scholar] [CrossRef]
- Shen, H.S.; Fu, X.K.; Bao, H.B.; Gong, B.W.; Chen, J.X. Synthesis and characterization of a new kind of immobilized Mn(salen) and catalytic epoxidation of styrene on the catalyst. Polym. Adv. Technol. 2009, 20, 77–83. [Google Scholar] [CrossRef]
- Zou, X.C.; Wang, C.; Wang, Y.; Shi, K.Y.; Wang, Z.M.; Li, D.W.; Fu, X.K. Chiral MnIII (Salen) Covalently Bonded on Modified ZPS-PVPA and ZPS-IPPA as Efficient Catalysts for Enantioselective Epoxidation of Unfunctionalized Olefins. Polymers 2017, 9, 108. [Google Scholar] [CrossRef]
- Tan, R.; Yin, D.H.; Yu, N.Y.; Jin, Y.; Zhao, H.H.; Yin, D.L. Ionic liquid-functionalized Salen Mn(III) complexes as tunable separation catalysts for enantioselective epoxidation of styrene. J. Catal. 2008, 255, 287–295. [Google Scholar] [CrossRef]
- Pietikäinen, P.; Haikarainen, A. Synthesis and catalytic activity of new chiral unsymmetrical Mn(III)-Schiff-base complexes containing salicylaldehyde and 1-(2-hydroxyphenyl)ketone units. J. Mol. Catal. A Chem. 2002, 180, 59–65. [Google Scholar] [CrossRef]
- Yao, X.Q.; Chen, H.L.; Lv, W.R.; Pan, G.Z.; Hu, X.Q.; Zheng, Z. Enantioselective epoxidation of olefins catalyzed by two novel chiral poly-salen-Mn(III) complexes. Tetrahedron Lett. 2000, 41, 10267–10270. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, N.H.; Jacobsen, E.N. Non-Stereospecific Mechanisms in Asymmetric Addition to Alkenes Result in Enantiodifferentiation after the First Irreversible Step. J. Am. Chem. Soc. 1994, 116, 425–426. [Google Scholar] [CrossRef]
- Wu, X.J.; Ma, X.B.; Ji, Y.L.; Wang, Q.; Jia, X.; Fu, X.K. Synthesisand characterizationof a novel type of self-assembled chiral zirconium phosphonates and its application for heterogeneous asymmetriccatalysis. J. Mol. Catal. A Chem. 2007, 265, 316–322. [Google Scholar] [CrossRef]










| Entry | Catalyst | Oxidant | Conv (%(h)) b | Sele (%) c | Ee (%) c |
|---|---|---|---|---|---|
| 1 | Chiral MnIII (salen)Cl | m-CPBA/NMO | 99.9(1) | 98.7 | 46.2 d |
| 2 | Catalyst 1 | m-CPBA/NMO | 92.6(3) | 91.7 | 53.3 d |
| 3 | Catalyst 2 | m-CPBA/NMO | 93.8(3) | 94.3 | 57.9 d |
| 4 | Catalyst 3 | m-CPBA/NMO | 95.1(3) | 99.1 | 63.9 d |
| 5 | Catalyst 3 | m-CPBA | 69.6(3) | 81.7 | 3.90 d |
| 6 | ZAPS-PVPA | m-CPBA | 12.3(3) | - | - |
| Entry | Catalyst mol% | Conv (%(h)) b | Sele (%) c | Ee (%) c |
|---|---|---|---|---|
| 1 | 0.5 | 35.6(3) | 78.3 | 49.6 d |
| 2 | 1 | 50.9(3) | 80.1 | 56.6 d |
| 3 | 2 | 82.6(3) | 88.7 | 60.3 d |
| 4 | 3 | 95.0(3) | 96.9 | 64.7 d |
| 5 | 4 | 95.1(3) | 97.6 | 63.1 d |
| 6 | 5 | 95.1(3) | 99.1 | 63.9 d |
| 7 | No catalyst | 7.9(3) | - | - |
| Entry | Solvent | Conv (%(h)) b | Sele (%) c | Ee (%) c |
|---|---|---|---|---|
| 1 | Dichloromethane | 95.0(3) | 96.9 | 64.7 d |
| 2 | n-Hexane | 41.9(3) | 27.6 | 13.9 d |
| 3 | ethanol | 40.1 | 18.6 | 15.5 d |
| 4 | Ethyl acetate | 89.9 | 83.6 | 41.0 d |
| 5 | Acetone | 83.2 | 80.6 | 38.2 d |
| 6 | Acetonitrile | 90.6 | 91.8 | 31.5 d |
| Entry | Temperature (°C) | Conv (%(h)) b | Sele (%) c | Ee (%) c |
|---|---|---|---|---|
| 1 | 0 | 95.0(3) | 96.9 | 64.7 d |
| 2 | −20 | 86.8(2) | 85.2 | 65.2 d |
| 3 | −40 | 79.2(2) | 86.3 | 67.8 d |
| 5 | −78 | 70.6(1) | 88.1 | 73.9 d |
| Run | Conv (%(h)) b | Sele (%) c | Ee (%) c |
|---|---|---|---|
| 1 | 95.0(3) | 96.9 | 64.7 d |
| 2 | 95.0(3) | 93.8 | 62.9 d |
| 3 | 94.1(3) | 89.6 | 62.1 d |
| 4 | 92.6(3) | 81.6 | 60.1 d |
| 5 | 91.2(3) | 71.8 | 58.7 d |
| 6 | 85.8(3) | 64.6 | 55.1 d |
| 7 | 80.8(3) | 50.9 | 51.3 d |
| 8 | 66.8(3) | 43.2 | 48.1 d |
| 9 | 93.2(3) | 86.0 | 54.0 d |
| 10 | 92.8(3) | 85.1 | 52.3 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Wang, Y.; Wang, C.; Shi, K.; Ren, Y.; Zhao, X. Chiral MnIII (Salen) Immobilized on Organic Polymer/Inorganic Zirconium Hydrogen Phosphate Functionalized with 3-Aminopropyltrimethoxysilane as an Efficient and Recyclable Catalyst for Enantioselective Epoxidation of Styrene. Polymers 2019, 11, 212. https://doi.org/10.3390/polym11020212
Zou X, Wang Y, Wang C, Shi K, Ren Y, Zhao X. Chiral MnIII (Salen) Immobilized on Organic Polymer/Inorganic Zirconium Hydrogen Phosphate Functionalized with 3-Aminopropyltrimethoxysilane as an Efficient and Recyclable Catalyst for Enantioselective Epoxidation of Styrene. Polymers. 2019; 11(2):212. https://doi.org/10.3390/polym11020212
Chicago/Turabian StyleZou, Xiaochuan, Yue Wang, Cun Wang, Kaiyun Shi, Yanrong Ren, and Xin Zhao. 2019. "Chiral MnIII (Salen) Immobilized on Organic Polymer/Inorganic Zirconium Hydrogen Phosphate Functionalized with 3-Aminopropyltrimethoxysilane as an Efficient and Recyclable Catalyst for Enantioselective Epoxidation of Styrene" Polymers 11, no. 2: 212. https://doi.org/10.3390/polym11020212