Synthesis and Study of Zinc Orotate and Its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of ZnOr2
2.3. Preparation of PVC Samples
2.4. Evaluation of Stabilizing Efficiency
2.4.1. Congo Red Test
2.4.2. Discoloration Test
2.4.3. Thermogravimetric Analysis
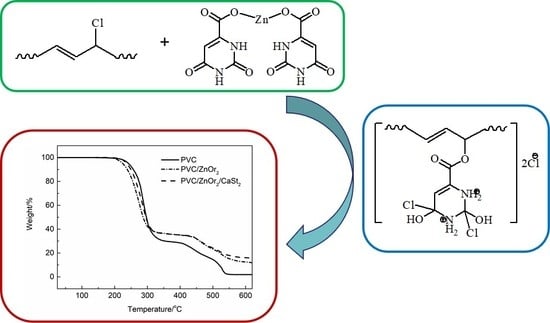
2.4.4. Investigation of the Mechanism of ZnOr2 for Stabilizing PVC
3. Results
3.1. Characterization of ZnOr2
3.2. Thermal Stability of ZnOr2 Stabilized PVC
 ) group in ZnOr2. This feature indicates that chemical bonds form between the orotate anion in ZnOr2 and the degraded polymeric chains during the stabilization process. Moreover, the FTIR spectrum of the purified PVC/ZnOr2 sample after ageing for 40 min is shown in Figure 7b. It can be seen that this fresh peak becomes weak and cannot be observed after being aged for 40 min [28]. These results suggest that the (
) group in ZnOr2. This feature indicates that chemical bonds form between the orotate anion in ZnOr2 and the degraded polymeric chains during the stabilization process. Moreover, the FTIR spectrum of the purified PVC/ZnOr2 sample after ageing for 40 min is shown in Figure 7b. It can be seen that this fresh peak becomes weak and cannot be observed after being aged for 40 min [28]. These results suggest that the (  ) group in ZnOr2 may play a major role in suppressing the thermal degradation of PVC.
) group in ZnOr2 may play a major role in suppressing the thermal degradation of PVC.3.3. Influence of Mixed Thermal Stabilizers on Thermal Stability of PVC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Avalos, A.S.; Hakkarainen, M.; Odelius, K. Superiorly plasticized PVC/PBSA blends through crotonic and acrylic acid functionalization of PVC. Polymers 2017, 9, 84. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Rabie, S.T.; Mohamed, R.R. Novel antimicrobial and antitumor organic thermal stabilizers for rigid Poly (vinyl chloride). J. Therm. Anal. Calorim. 2012, 109, 1503–1513. [Google Scholar] [CrossRef]
- Shnawa, H.A.; Jahani, Y.; Khalaf, M.N.; Taobi, A.H. The potential of tannins as thermal co-stabilizer additive for polyvinyl chloride. J. Therm. Anal. Calorim. 2016, 123, 1253–1261. [Google Scholar] [CrossRef]
- Shnawa, H.A. Thermal stabilization of polyvinyl chloride with traditional and naturally derived antioxidant and thermal stabilizer synthesized from tannins. J. Therm. Anal. Calorim. 2017, 129, 789–799. [Google Scholar] [CrossRef]
- Wu, B.Z.; Wang, Y.T.; Chen, S.; Wang, M.Y.; Ma, M.; Shi, Y.Q.; Wang, X. Bis-uracil based high efficient heat stabilizers used in super transparent soft poly (vinyl chloride). Polym. Degrad. Stab. 2018, 149, 143–151. [Google Scholar] [CrossRef]
- Xie, L.H.; Li, D.G.; Fu, M.; Zhang, J.; Zhang, L.P.; Zhang, Y.L.; Zhao, P.P. Study on lanthanum-pentaerythritol alkoxide as a thermal stabilizer for rigid polyvinyl chloride. J. Vinyl Addit. Technol. 2017, 23, 55–61. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.X.; Huang, X.L.; Yi, L. Zinc Maleate and Calcium Stearate as a Complex Thermal Stabilizer for Poly(vinyl chloride). J. Vinyl Addit. Technol. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Farag, Z.R.; Mohamed, N.A. Thermal Degradation Behavior of poly(vinyl chloride) in the Presence of poly(glycidyl methacrylate). J. Appl. Polym. Sci. 2008, 110, 2205–2210. [Google Scholar] [CrossRef]
- Zeddam, C.; Belhaneche-Bensemra, N. Application of FT-IR spectroscopy to the specific migration study of an organotin heat stabilizer from rigid poly(vinyl chloride) into food simulants. Polimery 2011, 56, 657–661. [Google Scholar] [CrossRef]
- Liu, J.P.; Shang, H.Z.; Zheng, Y.B.; Song, X. Synthesis and evaluation of poly (dioctyltin maleate-styrene-methyl acrylate) as a stabilizer for poly(vinyl chloride). J. Appl. Polym. Sci. 2009, 113, 1216–1222. [Google Scholar] [CrossRef]
- Bjorn, A.; Horsing, M.; Karlsson, A.; Mersiowsky, I.; Ejlertsson, J. Impacts of temperature on the leaching of organotin compounds from poly(vinyl chloride) plastics—A study conducted under simulated landfill conditions. J. Vinyl Addit. Technol. 2007, 13, 176–188. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.H.; Jiang, J.C.; Xia, J.L.; Li, M. Binary amide-containing tung-oil-based Ca/Zn stabilizers: Effects on thermal stability and plasticization performance of poly(vinyl chloride) and mechanism of thermal stabilization. Polym. Degrad. Stab. 2017, 143, 106–117. [Google Scholar] [CrossRef]
- Fang, L.; Song, Y.H.; Zhu, X.N.; Chen, S.H.; Du, P.H.; Zheng, Q.A. Influence of lanthanum stearate and calcium/zinc stabilizers on stabilization efficiency of dibutyltin dilaurate to polyvinyl chloride. Chin. J. Polym. Sci. 2010, 28, 637–645. [Google Scholar] [CrossRef]
- Wang, M.; Xia, J.L.; Jiang, J.C.; Li, S.H.; Huang, K.; Mao, W.; Li, M. A novel liquid Ca/Zn thermal stabilizer synthesized from tung-maleic anhydride and its effects on thermal stability and mechanical properties of PVC. Polym. Degrad. Stab. 2016, 133, 136–143. [Google Scholar] [CrossRef]
- Huanzhang, C.H.E.N.; Hua, L.I.; Hong, L.I. Study on preparation and performance of zinc cyanurate thermal stabilizer for PVC. J. Hebei Univ. Sci. Technol. 2016, 37, 33–38. [Google Scholar]
- Xu, X.P.; Chen, S.; Tang, W.; Qu, Y.J.; Wang, X. Investigation of basic zinc cyanurate as a novel thermal stabilizer for poly(vinyl chloride) and its synergistic effect with calcium stearate. Polym. Degrad. Stab. 2014, 99, 211–218. [Google Scholar] [CrossRef]
- Zhu, L.Z.; Wu, Y.J.; Shentu, B.Q.; Weng, Z.X. Preparation and characterization of zinc-mannitol complexes as PVC thermal stabilizers with high efficiency. Polym. Degrad. Stab. 2016, 133, 399–403. [Google Scholar] [CrossRef]
- Li, S.M.; Yao, Y.W. Effect of thermal stabilizers composed of zinc barbiturate and calcium stearate for rigid poly(vinyl chloride). Polym. Degrad. Stab. 2011, 96, 637–641. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr.; Jennings, T.C.; Krauskopf, L.G. Plastics Additives Handbook, 6th ed.; Hanser Publishers: Munich, Germany, 2005; Chapter 3. [Google Scholar]
- Santamaria, E.; Edge, M.; Allen, N.S.; Harvey, H.B.; Mellor, M.; Orchison, J. New Insights into the Degradation Mechanism of Poly (Vinyl Chloride), Part (III): Implementation of New Costabilizers—Towards Heavy Metal Free Systems (HMFS). J. Appl. Polym. Sci. 2005, 96, 122–143. [Google Scholar] [CrossRef]
- Chen, S.; Xu, X.P.; Zhang, J.H.; Tang, W.; Qu, Y.J.; Wang, X. Efficiency and mechanism for the stabilizing action of N,N′-bis(phenylcarbamoyl)alkyldiamines as thermal stabilizers and co-stabilizers for poly(vinyl chloride). Polym. Degrad. Stab. 2014, 105, 178–184. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.W.; Xin, J.N.; Huang, K.; Li, S.H.; Wang, M.; Xia, J.L. Design of green zinc-based thermal stabilizers derived from tung oil fatty acid and study of thermal stabilization for PVC. J. Appl. Polym. Sci. 2017, 134, 44679. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.C.; Zhang, J.W.; Yang, X.H.; Zhang, Y.; Li, S.H.; Song, J.; Huang, K.; Xia, J.L. Preparation of a new liquid thermal stabilizer from rosin and fatty acid and study of the properties of the stabilized PVC. Polym. Degrad. Stab. 2014, 109, 129–136. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.D.; Wu, Y.X.; Li, K.S. Synergistic effect of complexes of ethylenediamine double maleamic acid radical and lanthanum(III) with pentaerythritol on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2017, 140, 176–193. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.W.; Huang, K.; Li, S.H.; Jiang, J.C.; Xia, J.L. Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: Preparation and thermal stabilization for PVC. RSC Adv. 2014, 4, 63576–63585. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.H.; Jiang, J.C.; Xia, J.L.; Li, M. Influence of Zeolitic imidazolate framework-8 on the thermal stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2018, 149, 112–118. [Google Scholar] [CrossRef]
- Wu, B.Z.; Wang, Y.T.; Chen, S.; Wang, M.Y.; Ma, M.; Shi, Y.Q.; Wang, X. Stability, mechanism and unique “zinc burning” inhibition synergistic effect of zinc dehydroacetate as thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stab. 2018, 152, 228–234. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Chen, S.; Ma, M.; Wu, B.Z.; Ying, J.; Xu, X.P.; Wang, X. Highly efficient and antibacterial zinc norfloxacin thermal stabilizer for poly(vinyl chloride). RSC Adv. 2016, 6, 97491–97502. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.H.; Jiang, J.C.; Xia, L.; Li, S.H.; Li, M. Excellent hydroxyl and nitrogen rich groups-containing tung-oil-based Ca/Zn and polyol stabilizers for enhanced thermal stability of PVC. Thermochim. Acta 2017, 658, 84–92. [Google Scholar] [CrossRef]
- Zhang, D.F.; Yao, S.; Li, S.; Wang, J.; Yao, Y.W. A novel La-containing additive for the long-term thermal stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2017, 144, 187–194. [Google Scholar] [CrossRef]
- Khaleghi, M.; Didehban, K.; Shabanian, M. Effect of new melamine-terephthaldehyde resin modified graphene oxide on thermal and mechanical properties of PVC. Polym. Test. 2017, 63, 382–391. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.C.; Zhang, J.; Zhu, W.Q.; Tian, T.S. Remarkably improved toughness and thermal stability of poly (vinyl chloride) (PVC)/poly (alpha-methyl-styrene-acrylonitrile) (alpha-MSAN) blend with the assistance of two impact modifiers. Polym. Test. 2016, 51, 1–5. [Google Scholar] [CrossRef]
- Mohamed, N.A. Biologically active maleimido aromatic 1,3,4-oxadiazole derivatives evaluated thermogravimetrically as stabilizers for rigid PVC. J. Therm. Anal. Calorim. 2018, 131, 2535–2546. [Google Scholar] [CrossRef]
- Jiang, P.P.; Song, Y.Y.; Dong, Y.M.; Yan, C.R.; Liu, P. Zinc glycerolate with lanthanum stearate to inhibit the thermal degradation of poly(vinyl chloride). J. Appl. Polym. Sci. 2013, 127, 3681–3686. [Google Scholar] [CrossRef]
- Du, Y.G.; Gao, J.G.; Liu, X.Q.; Yang, J.B. Influence of Nano-CeO2 as Co-Stabilizer on the Thermal Stabilization Efficiency of Novel Calcium/Zinc Soap Stabilizers for Poly(vinyl chloride). J. Vinyl Addit. Technol. 2014, 20, 243–249. [Google Scholar] [CrossRef]
- Ye, F.; Guo, X.J.; Zhan, H.H.; Lin, J.X.; Lou, W.C.; Ma, X.T.; Wang, X. The synergistic effect of zinc urate with calcium stearate and commercial assistant stabilizers for stabilizing poly(vinyl chloride). Polym. Degrad. Stab. 2018, 156, 193–201. [Google Scholar] [CrossRef]
- Li, M.; Duan, C.; Wang, H.O.; Liu, Z.G.; Wang, M.T.; Hu, Y.H. Lanthanum histidine with pentaerythritol and zinc stearate as thermal stabilizers for poly(vinyl chloride). J. Appl. Polym. Sci. 2016, 133, 42878. [Google Scholar] [CrossRef]


















| PVC/Stabilizer | T5% | T10% | Tr | Wf (%) |
|---|---|---|---|---|
| PVC | 242.7 | 257.0 | 287.6 | 29.6 |
| PVC/ZnOr2 | 224.6 | 238.5 | 273.6 | 35.7 |
| PVC/ZnOr2/CaSt2 | 238.5 | 249.5 | 288.5 | 35.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, F.; Ye, Q.; Zhan, H.; Ge, Y.; Ma, X.; Xu, Y.; Wang, X. Synthesis and Study of Zinc Orotate and Its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride). Polymers 2019, 11, 194. https://doi.org/10.3390/polym11020194
Ye F, Ye Q, Zhan H, Ge Y, Ma X, Xu Y, Wang X. Synthesis and Study of Zinc Orotate and Its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride). Polymers. 2019; 11(2):194. https://doi.org/10.3390/polym11020194
Chicago/Turabian StyleYe, Feng, Qiufeng Ye, Haihua Zhan, Yeqian Ge, Xiaotao Ma, Yingying Xu, and Xu Wang. 2019. "Synthesis and Study of Zinc Orotate and Its Synergistic Effect with Commercial Stabilizers for Stabilizing Poly(Vinyl Chloride)" Polymers 11, no. 2: 194. https://doi.org/10.3390/polym11020194