Removal of Pb(II) Ions Using Polymer Inclusion Membranes Containing Calix[4]resorcinarene Derivative as Ion Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis
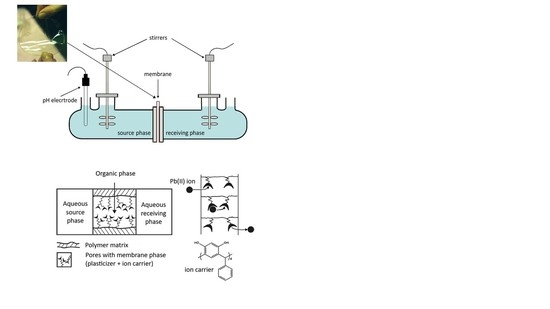
2.3. Preparation of PIMs
2.4. Transport Studies
3. Results and Discussion
3.1. Effect of Acidity of the Source Phase
3.2. Effect of the Type of Agents in the Receiving Phase
3.3. Effect of Carrier Concentration
3.4. Effect of Plasticizer Amount in the Membrane
3.5. Membrane Stability and Reusability
3.6. Effect of Temperature on the Transport Process
3.7. Selective Removal of Pb(II) from the Battery Industry Effluent
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kocaoba, S. Comparison of Amberlite IR 120 and dolomite’s performances for removal of heavy metals. J. Hazard. Mater. 2007, 147, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Weng, X.; Ma, L.; Sarkar, B.; Chen, Z. Mechanistic insights into Pb(II) removal from aqueous solution by green reduced graphene oxide. J. Colloid Interface Sci. 2019, 550, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, C.A.; Nowik-Zajac, A.; Kurcok, P. Application of O-phenylacetyl β-CD as a carrier for selective transport of lead(II) across polymer inclusion membranes. Desalin. Water Treat. 2014, 52, 4257–4265. [Google Scholar] [CrossRef]
- Awual, M.R. Novel conjugated hybrid material for efficient lead(II) capturing from contaminated wastewater. Mater. Sci. Eng. C 2019, 101, 686–695. [Google Scholar] [CrossRef]
- Kavak, D.; Demir, M.; Başsayel, B.; Anagün, A.S. Factorial experimental design for optimizing the removal of lead ions from aqueous solutions by cation exchange resin. Desalin. Water Treat. 2013, 51, 1712–1719. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W.; Girek, T. Modified cyclodextrin polymers as selective ion carriers for Pb(II) separation across plasticized membranes. J. Membr. Sci. 2008, 310, 312–320. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Immobilized materials for removal of toxic metal ions from surface/groundwaters and aqueous waste streams. Environ. Sci. Proc. Impacts 2016, 4, 429–444. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowska, J.; Kozlowski, C.; Trochimczuk, A. Sorption of Pb(II), Cd(II) and Zn(II) performed with the use of carboxyphenylresorcinarene-impregnated Amberlite XAD-4 resin. Desalin. Water Treat. 2014, 52, 314–323. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Cho, Y.; Cattrall, R.W.; Kolev, S.D. A novel polymer inclusion membrane based method for continuous clean-up of thiocyanate from gold mine tailings water. J. Hazard. Mater. 2018, 341, 297–303. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Kozlowski, C.A. Facilitated transport of metal ions through composite and polymer inclusion membranes. Desalination 2006, 198, 140–148. [Google Scholar] [CrossRef]
- Upitis, A.; Peterson, J.; Lukey, C.; Nghiem, L.D. Metallic ion extraction using polymer inclusion membranes (PIMs): Optimising physical strength and extraction rate. Desalin. Water Treat. 2009, 6, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Benosmane, N.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Selective transport of metal ions across polymer inclusion membranes (PIMs) containing calix[4]resorcinarenes. Sep. Purif. Technol. 2009, 65, 211–219. [Google Scholar] [CrossRef]
- Sliwa, W.; Kozlowski, C. Calixarenes and Resorcinarenes. Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.M.; Yang, Y.F.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Ma, H.M.; Wang, Z.H.; Zheng, Q.Y.; Wen, L.Q.; Ma, Q.L.; Su, M.H.; Huang, Z.T. Liquid membrane transport of transition metal ions with calix[4]crowns. Chem. J. Chin. Univ. 2001, 22, 1315–1318. [Google Scholar]
- Alpoguz, K.H.; Shahabuddin, M.; Mustafa, E.; Mustafa, Y. Transport of Hg2+ through bulk liquid membrane using a bis-calix[4]arene nitrile derivative as carrier: Kinetic analysis. New J. Chem. 2002, 26, 477–480. [Google Scholar] [CrossRef]
- Ulewicz, M.; Lesinska, U.; Bochenska, M.; Walkowiak, W. Facilitated transport of Zn(II), Cd(II) and Pb(II) ions through polymer inclusion membranes with calix[4]-crown-6 derivatives. Sep. Purif. Technol. 2007, 54, 299–306. [Google Scholar] [CrossRef]
- Zaghbani, A.; Tayeb, R.; Bonnamour, I.; Felix, C.; Vocanson, F.; Lamartine, R.; Dhahbi, M.; Seta, P. Affinity membranes for the extraction of Cd2+ metal ions by facilitated transport ensured by a new thiacalix[4]arene complexing agent incorporated in supported liquid membranes (SLM). J. Membr. Sci. 2005, 258, 5–7. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Ruse, E.; Nechifor, G.; Serban, B. Calixarene-transportori in membrane lichide III. Transportul cationilor Pb2+ prin membrane lichide cu-tio-calix[4]arene. Rev. Chim. Buchar. 2002, 1, 20–26. [Google Scholar]
- Sap, A.; Tabakci, B.; Yilmaz, A. Calix[4]arene-based Mannich and Schiff bases as versatile receptors for dichromate anion extraction: Synthesis and comparative studies. Tetrahedron 2012, 68, 8739–8745. [Google Scholar] [CrossRef]
- Kozlowski, C.A. Kinetics of chromium(VI) transport from mineral acids across cellulose triacetate (CTA) plasticized membranes immobilized by tri-n-octylamine. Ind. Eng. Chem. Res. 2007, 46, 5420–5428. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Asfari, Z.; Bohmer, V.; Harroweld, J.; Vicens, J.; Saadioui, M. Calixarenes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Kim, J.S.; Kim, S.K.; Ko, J.W.; Kim, E.T.; Yu, S.H.; Cho, M.H.; Kwon, S.G.; Lee, E.H. Selective transport of cesium ion in polymeric CTA membrane containing calixcrown ethers. Talanta 2000, 52, 1143–1148. [Google Scholar] [CrossRef]
- Ulewicz, M.; Sadowska, K.; Biernat, J.F. Facilitated transport of Zn(II), Cd(II) and Pb(II) across polymer inclusion membranes doped with imidazole azocrown ethers. Desalination 2007, 214, 352–364. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Kozlowska, J. PNP-16-crown-6 derivatives as ion carriers for Zn(II), Cd(II) and Pb(II) transport across polymer inclusion membranes. J. Membr. Sci. 2009, 326, 215–221. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer inclusion membranes (PIMs) doped with alkylimidazole and their application in the separation of non-ferrous metal ions. Polymers 2019, 11, 1780. [Google Scholar] [CrossRef] [Green Version]
- Lazarova, Z.; Boyadzhiev, L. Kinetic aspects of copper(II) transport across liquid membrane containing LIX-860 as a carrier. J. Membr. Sci. 1993, 78, 239–245. [Google Scholar] [CrossRef]










| Receiving agent | Percentage of Pb(II) transported into the receiving phase | Percentage of Pb(II) remaining in the source phase | Flux (µmol/m2s) |
|---|---|---|---|
| 0.1 M HCl | 94 | 5 | 9.7 |
| 0.1 M HNO3 | 65 | 10 | 5.6 |
| 0.1 M CH3COOH | 44 | 20 | 4.2 |
| Distilled water | 5 | 9 | 1.8 |
| Membrane | Membrane composition | Membrane thickness (µm) | Pb(II) initial flux J·106 (mol·s−1·m−2) | Ref. |
|---|---|---|---|---|
| PIM | CTA/azacrown ethers/o-nitrophenyl pentyl ether (o-NPPE) | 30–35 | 1.79 | [27] |
| PIM | CTA/PNP-16-crown-6 derivatives/o-NPPE | 25 | 2.24 | [28] |
| PIM | CTA/calix[4]crown-6 derivatives/o-NPPE | 35 | 5.56 | [19] |
| PIM | CTA/β-CD polymer/o-NPPE | 28 | 1.25 | [6] |
| PIM | CTA/calix[4]resorcinarene/o-NPOE | 25 | 9.7 | This study |
| Temperature (K) | k·104 (s−1) | P·106 (m/s) | J·106 (mol/m2s) | D·109 (m2/s) | RF (%) | Transport time (h) |
|---|---|---|---|---|---|---|
| 288 | 3.49 | 9.45 | 8.5 | 4.9 | 91 | 6 |
| 293 | 3.71 | 10.63 | 9.7 | 5.1 | 94 | 6 |
| 298 | 3.84 | 11.23 | 11.9 | 5.6 | 96 | 6 |
| 303 | 4.09 | 12.46 | 13.6 | 6.1 | 97 | 5 |
| 308 | 4.44 | 14.67 | 14.2 | 6.5 | 98 | 5 |
| 313 | 5.8 | 18.03 | 16.4 | 7.2 | 99 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C.A. Removal of Pb(II) Ions Using Polymer Inclusion Membranes Containing Calix[4]resorcinarene Derivative as Ion Carrier. Polymers 2019, 11, 2111. https://doi.org/10.3390/polym11122111
Zawierucha I, Nowik-Zajac A, Kozlowski CA. Removal of Pb(II) Ions Using Polymer Inclusion Membranes Containing Calix[4]resorcinarene Derivative as Ion Carrier. Polymers. 2019; 11(12):2111. https://doi.org/10.3390/polym11122111
Chicago/Turabian StyleZawierucha, Iwona, Anna Nowik-Zajac, and Cezary A. Kozlowski. 2019. "Removal of Pb(II) Ions Using Polymer Inclusion Membranes Containing Calix[4]resorcinarene Derivative as Ion Carrier" Polymers 11, no. 12: 2111. https://doi.org/10.3390/polym11122111