Poly(Acrylonitrile–Butadiene–Styrene) as a Special β-Nucleating Agent on the Toughness of Isotactic Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. Dynamic Rheological Properties
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Wide-Angle X-Ray Diffraction (WAXD)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Mechanical Properties Measurements
3. Results and Discussion
3.1. Dynamic Rheological Behavior
3.2. Crystallization and Melting Behavior
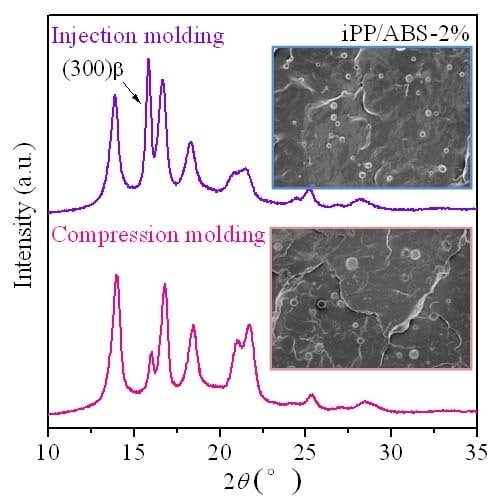
3.3. Crystalline Structure and Phase Morphology
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padden, F.J., Jr.; Keith, H.D. Spherulitic crystallization in polypropylene. J. Appl. Phys. 1959, 30, 1479–1484. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Cobbold, A.J. The β crystalline form of isotactic polypropylene. J. Polym. Sci. Part C Polym. Lett. 1968, 6, 539–546. [Google Scholar] [CrossRef]
- Kardos, J.L.; Christiansen, A.W.; Baer, E. Structure of pressure-crystallized polypropylene. J. Polym. Sci. Part B Polym. Phys. 1966, 4, 777–788. [Google Scholar] [CrossRef]
- Piccarolo, S.; Saiu, M.; Brucato, V.; Titomanlio, G. Crystallization of polymer melts under fast cooling. II. High-purity iPP. J. Appl. Polym. Sci. 1992, 46, 625–634. [Google Scholar] [CrossRef]
- Fujiyama, M. Structures and properties of injection moldings of β-crystal nucleator-added polypropylenes: Part 1 Effect of β-crystal nucleator content. Int. Polym. Proc. 1995, 10, 172–178. [Google Scholar] [CrossRef]
- Varga, J. Supermolecular structure of isotactic polypropylene. J. Mater. Sci. 1992, 27, 2557–2579. [Google Scholar] [CrossRef]
- Luo, F.; Geng, C.; Wang, K.; Deng, H.; Chen, F.; Fu, Q.; Na, B. New understanding in tuning toughness of β-polypropylene: The role of β-nucleated crystalline morphology. Macromolecules 2009, 42, 9325–9331. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Wang, C.; Jiang, J.; Li, G.; Mai, K. Non-isothermal crystallization kinetics and morphology of wollastonite-filled β-isotactic polypropylene composites. J. Therm. Anal. Calorim. 2014, 115, 675–688. [Google Scholar] [CrossRef]
- Wang, M.; Lin, L.; Peng, Q.; Ou, W.; Li, H. Crystallization and mechanical properties of isotactic polypropylene/calcium carbonate nanocomposites with a stratified distribution of calcium carbonate. J. Appl. Polym. Sci. 2014, 131, 39632. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, S.; Xin, Z. Influence of a novel β-nucleating agent on the structure, mechanical properties, and crystallization behavior of isotactic polypropylene. J. Thermoplast. Compos. Mater. 2015, 28, 610–629. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Y.; Zhang, Y. Crystallization, melting behavior, and morphology of PP/Novolac blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3288–3303. [Google Scholar] [CrossRef]
- Hu, J.S.; Kong, B.; Chao, C.Y.; Sun, J. Study on liquid-crystalline copolymers as a new β-nucleator to induce crystallization structure of isotactic polypropylene. Chem. J. Chin. Univ. 2009, 30, 1253–1255. [Google Scholar]
- Hu, J.S.; Kong, B.; Sun, J.; Lv, H.L.; Li, H. Effect of nematic liquid crystalline copolymer as a β-form nucleator on crystallization structure and thermal properties of isotactic polypropylene. Acta Polym. Sin. 2010, 9, 1100–1107. [Google Scholar] [CrossRef]
- Sun, J.; Hu, J.S.; Chao, C.Y.; Guo, Z.X.; Qi, Y. Influence of liquid crystalline copolysiloxane as a β-form nucleator on crystallization structure and morphology of isotactic polypropylene. Acta Chim. Sin. 2010, 68, 1003–1009. [Google Scholar]
- Sun, J.; Li, Q.; Yao, X.J.; Hu, J.S.; Qi, Y. A nematic liquid crystalline polymer as highly active novel β-nucleating agent for isotactic polypropylene. J. Mater. Sci. 2013, 48, 4032–4040. [Google Scholar] [CrossRef]
- Sun, J.; Hu, J.S.; Guo, Z.X.; Qi, Y. Study on side-chain liquid–crystalline copolymer as a new β-nucleating agent to induce phase behavior of isotactic polypropylene. Colloid Polym. Sci. 2013, 291, 735–742. [Google Scholar] [CrossRef]
- Yang, R.; Ding, L.; Chen, W.; Chen, L.; Zhang, X.; Li, J. Chain folding in main-chain liquid crystalline polyester with strong π–π interaction: An efficient β-nucleating agent for isotactic polypropylene. Macromolecules 2017, 50, 1610–1617. [Google Scholar] [CrossRef]
- Yang, R.; Ding, L.; Chen, W.; Chen, L.; Zhang, X.; Li, J. Nonisothermal crystallization, melting behaviors, and mechanical properties of isotactic polypropylene nucleated with a liquid crystalline polymer. Ind. Eng. Chem. Res. 2018, 57, 2083–2093. [Google Scholar] [CrossRef]
- Su, Z.Q.; Dong, M.; Guo, Z.X.; Yu, J. Study of polystyrene and acrylonitrile-styrene copolymer as special β-nucleating agents to induce the crystallization of isotactic polypropylene. Macromolecules 2007, 40, 4217–4224. [Google Scholar] [CrossRef]
- Su, Z.Q.; Chen, X.N.; Yu, Z.Z.; Zhang, L. Morphological distribution of polymeric nucleating agents in injection-molded isotactic polypropylene plates and its influence on nucleating efficiency. J. Appl. Polym. Sci. 2009, 111, 786–793. [Google Scholar] [CrossRef]
- Cao, L.; Dong, M.; Zhang, A.; Liu, Y.; Yang, W.; Su, Z.; Chen, X. Morphologies and crystal structures of styrene-acrylonitrile/isotactic polypropylene ultrafine fibers fabricated by melt electrospinning. Polym. Eng. Sci. 2013, 53, 2674–2682. [Google Scholar] [CrossRef]
- Phillips, A.W.; Zhu, P.W.; Edward, G. Polystyrene as a versatile nucleating agent for polypropylene. Polymer 2010, 51, 1599–1607. [Google Scholar] [CrossRef]
- Phillips, A.W.; Bhatia, A.; Zhu, P.W.; Edward, G. Shish formation and relaxation in sheared isotactic polypropylene containing nucleating particles. Macromolecules 2011, 44, 3517–3528. [Google Scholar] [CrossRef]
- Varga, J.; Karger-Kocsis, J. Rules of supermolecular structure formation in sheared isotactic polypropylene melts. J. Polym. Sci. Part B Polym. Phys. Ed. 1996, 34, 657–670. [Google Scholar] [CrossRef]
- Spina, R.; Spekowius, M.; Hopmann, C. Simulation of crystallization of isotactic polypropylene with different shear regimes. Thermochim. Acta 2018, 659, 44–54. [Google Scholar] [CrossRef]
- Byelov, D.; Panine, P.; Remerie, K.; Biemond, E.; Alfonso, G.C.; de Jeu, W.H. Crystallization under shear in isotactic polypropylene containing nucleators. Polymer 2008, 49, 3076–3083. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, X.; Lin, J.; Chen, Q. Compatibilization of polypropylene/poly (acrylonitrile-butadiene-styrene) blends by polypropylene-graft-cardanol. J. Appl. Polym. Sci. 2015, 132, 41315. [Google Scholar] [CrossRef]
- Lee, H.G.; Sung, Y.T.; Lee, Y.K.; Kim, W.N.; Yoon, H.G.; Lee, H.S. Effects of PP-g-MAH on the mechanical, morphological and rheological properties of polypropylene and poly (acrylonitrile-butadiene-styrene) blends. Macromol. Res. 2009, 17, 417–423. [Google Scholar] [CrossRef]
- Khare, R.A.; Bhattacharyya, A.R.; Kulkarni, A.R. Melt-mixed polypropylene/acrylonitrile-butadiene-styrene blends with multiwall carbon nanotubes: Effect of compatibilizer and modifier on morphology and electrical conductivity. J. Appl. Polym. Sci. 2011, 120, 2663–2672. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, Q.; Ma, F.; Jiang, Y. The effect of graft copolymers of maleic anhydride and epoxy resin on the mechanical properties and morphology of PP/ABS blends. J. Appl. Polym. Sci. 2014, 131, 40898. [Google Scholar] [CrossRef]
- Triantou, M.I.; Gavriel, M.; Sakellaris, P.; Tarantili, P.A. The effect of compatibilizers and organically modified nanoclay on the morphology and performance properties of poly (acrylonitrile-butadiene-styrene)/polypropylene blends. Polym. Eng. Sci. 2016, 56, 458–468. [Google Scholar] [CrossRef]
- Kum, C.K.; Sung, Y.T.; Kim, Y.S.; Lee, H.G.; Kim, W.N.; Lee, H.S.; Yoon, H.G. Effects of compatibilizer on mechanical, morphological, and rheological properties of polypropylene/poly (acrylonitrile-butadiene-styrene) blends. Macromol. Res. 2007, 15, 308–314. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, J.B.; Park, D.H.; Kim, W.N. Effects of accelerated aging and compatibilizers on the mechanical and morphological properties of polypropylene and poly (acrylonitrile-butadiene-styrene) blends. J. Appl. Polym. Sci. 2013, 127, 1032–1037. [Google Scholar] [CrossRef]
- Sung, Y.T.; Kim, Y.S.; Lee, Y.K.; Kim, W.N.; Lee, H.S.; Sung, J.Y.; Yoon, H.G. Effects of clay on the morphology of poly (acrylonitrile-butadiene-styrene) and polypropylene nanocomposites. Polym. Eng. Sci. 2007, 47, 1671–1677. [Google Scholar] [CrossRef]
- Hom, S.; Bhattacharyya, A.R.; Khare, R.A.; Kulkarni, A.R.; Saroop, M.; Biswas, A. PP/ABS blends with carbon black: Morphology and electrical properties. J. Appl. Polym. Sci. 2009, 112, 998–1004. [Google Scholar] [CrossRef]
- Khare, R.A.; Bhattacharyya, A.R.; Kulkarni, A.R.; Saroop, M.; Biswas, A. Influence of multiwall carbon nanotubes on morphology and electrical conductivity of PP/ABS blends. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2286–2295. [Google Scholar] [CrossRef]
- Shu, Q.; Zou, X.; Dai, W.; Fu, Z. Formation of β-iPP in isotactic polypropylene/acrylonitrile–butadiene–styrene blends: Effect of resin type, phase composition, and thermal condition. J. Macromol. Sci. Part B 2012, 51, 756–766. [Google Scholar] [CrossRef]
- Varga, J.; Garzó, G. The properties of polymer blends of the β-modification of polypropylene and an elastomer. Macromol. Mater. Eng. 1990, 180, 15–33. [Google Scholar]
- Grein, C.; Plummer, C.J.G.; Kausch, H.H.; Germain, Y.; Béguelin, P. Influence of β nucleation on the mechanical properties of isotactic polypropylene and rubber modified isotactic polypropylene. Polymer 2002, 43, 3279–3293. [Google Scholar] [CrossRef]
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Die Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Zipper, P.; Janosi, A.; Wrentschur, E. Scanning X-ray scattering of mouldings from semicrystalline polymers. J. Phys. IV 1993, 3, 33–36. [Google Scholar] [CrossRef]
- Liu, J.R.; Li, C.; Hu, F.M. Effect of polystyrenes with different architectures on the β-nucleating efficiency and toughening of isotactic polypropylene. Polym. Int. 2018, 67, 506–514. [Google Scholar] [CrossRef]
- Fujiyama, M.; Wakino, T.; Kawasaki, Y. Structure of skin layer in injection-molded polypropylene. J. Appl. Polym. Sci. 1988, 35, 29–49. [Google Scholar] [CrossRef]










| Samples | First Cooling Scan | Second Heating Scan | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g−1) | Tm,β (°C) | △Hm.β (J·g−1) | Tm,α (°C) | ΔHm.α (J·g−1) | ΔHm (J·g−1) | Xβ (%) | Xα (%) | Xall (%) | φβ (%) | |
| iPP | 111.9 | 107.0 | - | - | 164.2 | 95.2 | 95.2 | - | 53.8 | 53.8 | 0 |
| P-0.3A | 115.3 | 100.5 | 149.3 | 7.3 | 163.6 | 89.6 | 96.9 | 4.3 | 50.6 | 54.9 | 7.9 |
| P-0.7A | 115.0 | 99.6 | 148.6 | 17.2 | 163.3 | 86.7 | 103.9 | 10.2 | 49.0 | 59.2 | 17.2 |
| P-1A | 115.4 | 99.7 | 148.7 | 26.4 | 163.2 | 72.7 | 99.0 | 15.6 | 41.1 | 56.7 | 27.6 |
| P-2A | 115.5 | 98.1 | 150.0 | 52.0 | 163.4 | 52.9 | 104.9 | 30.9 | 29.9 | 60.7 | 50.8 |
| P-4A | 116.1 | 97.4 | 149.1 | 21.4 | 163.8 | 80.0 | 101.3 | 12.7 | 45.2 | 57.9 | 21.9 |
| Samples | Compression Molding | Injection Molding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xc (%) | A100 (%) | A130 (%) | C (%) | L110 (nm) | L040 (nm) | Xc (%) | A100 (%) | A130 (%) | C (%) | L110 (nm) | L040 (nm) | |
| iPP | 60.6 | 51.2 | 34.9 | 37.5 | 16.1 | 16.0 | 53.9 | 84.3 | 69.1 | 45.0 | 16.0 | 15.5 |
| P-0.3A | 70.3 | 50.5 | 34.3 | 36.9 | 16.0 | 16.1 | 55.5 | 80.0 | 67.7 | 44.4 | 16.6 | 15.8 |
| P-0.7A | 72.3 | 50.1 | 33.4 | 36.5 | 15.6 | 15.3 | 61.4 | 70.4 | 57.9 | 43.1 | 16.1 | 15.9 |
| P-1A | 78.0 | 49.1 | 32.4 | 34.6 | 16.2 | 15.7 | 65.3 | 61.7 | 45.6 | 41.6 | 15.5 | 14.9 |
| P-2A | 75.1 | 52.2 | 35.5 | 35.3 | 15.5 | 16.0 | 73.6 | 70.9 | 52.3 | 37.8 | 16.1 | 14.5 |
| P-4A | 61.5 | 52.3 | 38.5 | 41.1 | 16.0 | 15.8 | 54.7 | 82.4 | 70.0 | 41.9 | 16.1 | 15.0 |
| Blends | Compression Molding | Injection Molding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin Region | Core Region | |||||||||||
| Dn (μm) | DW (μm) | DV (μm) | PD | Dn (μm) | DW (μm) | DV (μm) | PD | Dn (μm) | DW (μm) | DV (μm) | PD | |
| P-0.3A | 1.109 | 1.149 | 1.215 | 1.096 | 0.863 | 0.899 | 0.901 | 1.044 | 0.882 | 0.918 | 0.922 | 1.045 |
| P-0.7A | 1.130 | 1.169 | 1.242 | 1.099 | 0.926 | 0.943 | 0.974 | 1.052 | 0.955 | 0.970 | 1.019 | 1.067 |
| P-1A | 1.149 | 1.234 | 1.385 | 1.205 | 0.979 | 1.097 | 1.069 | 1.092 | 1.055 | 1.128 | 1.161 | 1.100 |
| P-2A | 1.379 | 1.471 | 1.738 | 1.260 | 1.012 | 1.132 | 1.159 | 1.145 | 1.074 | 1.196 | 1.252 | 1.166 |
| P-4A | 2.376 | 2.689 | 3.169 | 1.334 | 1.528 | 1.419 | 1.815 | 1.188 | 1.567 | 1.769 | 1.995 | 1.273 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhu, X.; Cao, Z. Poly(Acrylonitrile–Butadiene–Styrene) as a Special β-Nucleating Agent on the Toughness of Isotactic Polypropylene. Polymers 2019, 11, 1894. https://doi.org/10.3390/polym11111894
Liu J, Zhu X, Cao Z. Poly(Acrylonitrile–Butadiene–Styrene) as a Special β-Nucleating Agent on the Toughness of Isotactic Polypropylene. Polymers. 2019; 11(11):1894. https://doi.org/10.3390/polym11111894
Chicago/Turabian StyleLiu, Jingru, Xinxu Zhu, and Zheng Cao. 2019. "Poly(Acrylonitrile–Butadiene–Styrene) as a Special β-Nucleating Agent on the Toughness of Isotactic Polypropylene" Polymers 11, no. 11: 1894. https://doi.org/10.3390/polym11111894