Wasted Ganoderma tsugae Derived Chitosans for Smear Layer Removal in Endodontic Treatment
Abstract
:1. Introduction
2. Materials and Methods
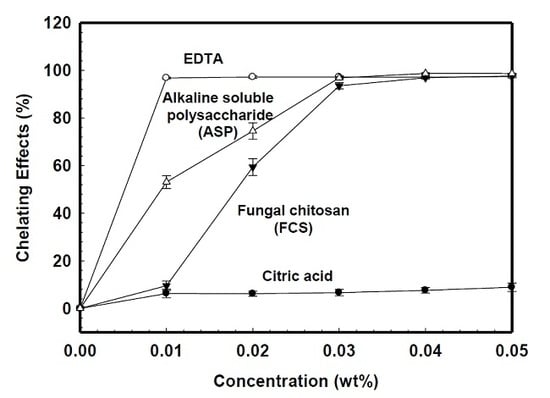
2.1. Chelating Ability
2.2. Smear Layer Removal
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Acid Etching Effect
4.2. Chelation Effect
4.3. Acid Etching Plus Chelation Effect
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alves, F.R.; Almeida, B.M.; Neves, M.A.; Moreno, J.O.; Rocas, I.N.; Siqueira, J.F., Jr. Disinfecting oval-shaped root canals: Effectiveness of different supplementary approaches. J. Endod. 2011, 37, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Jurič, I.B.; Anić, I. The Use of Lasers in Disinfection and Cleanliness of Root Canals: A Review. Acta Stomatol. Croat. 2014, 48, 6–15. [Google Scholar] [CrossRef] [PubMed]
- McComb, D.; Smith, D.C. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J. Endod. 1975, 1, 238–242. [Google Scholar] [CrossRef]
- Torabinejad, M.; Handysides, R.; Khademi, A.A.; Bakland, L.K. Clinical implications of the smear layer in endodontics: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.S.; Kim, J.R.; Ling, J.; Choi, K.K.; Pashley, D.H.; Tay, F.R. Review of contemporary irrigant agitation techniques and devices. J. Endod. 2009, 35, 791–804. [Google Scholar] [CrossRef]
- Arruda, M.; de Arruda, M.P.; de Carvalho-Junior, J.R.; de Souza-Filho, F.J.; Sousa-Neto, M.D.; de Freitas, G.C. Removal of the smear layer from flattened canals using different chemical substances. Gen. Dent. 2007, 55, 523–526. [Google Scholar]
- Jiang, L.M.; Lak, B.; Eijsvogels, L.M.; Wesselink, P.; van der Sluis, L.W. Comparison of the cleaning efficacy of different final irrigation techniques. J. Endod. 2012, 38, 838–841. [Google Scholar] [CrossRef]
- Harashima, T.; Takeda, F.H.; Kimura, Y.; Matsumoto, K. Effect of Nd:YAG laser irradiation for removal of intracanal debris and smear layer in extracted human teeth. J. Clin. Laser Med. Surg. 1997, 15, 131–135. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Gao, W. [Effect of different techniques in root canal preparation on coronal microleakage of endodontically treated teeth]. Hua Xi Kou Qiang Yi Xue Za Zhi 2012, 30, 522–525. [Google Scholar]
- Carvalho, A.S.; Camargo, C.H.; Valera, M.C.; Camargo, S.E.; Mancini, M.N. Smear layer removal by auxiliary chemical substances in biomechanical preparation: A scanning electron microscope study. J. Endod. 2008, 34, 1396–1400. [Google Scholar] [CrossRef]
- von der Fehr, F.R.; Nygaard-Östby, B. Effect of edtac and sulfuric acid on root canal dentine. Oral Surg. Oral Med. Oral Path. 1963, 16, 199–205. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final Report on the Safety Assessment of EDTA, Calcium Disodium EDTA, Diammonium EDTA, Dipotassium EDTA, Disodium EDTA, TEA-EDTA, Tetrasodium EDTA, Tripotassium EDTA, Trisodium EDTA, HEDTA, and Trisodium HEDTA. Int. J. Toxicol. 2002, 21, 95–142. [Google Scholar] [PubMed]
- Di Lenarda, R.; Cadenaro, M.; Sbaizero, O. Effectiveness of 1 mol L-1 citric acid and 15% EDTA irrigation on smear layer removal. Int. Endod. J. 2000, 33, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zalloum, H.M.; Mubarak, M.S. Chitosan and chitosan derivatives as chelating agents. In Natural Polymers, Biopolymers, Biomaterials, and Their Composites, Blends, and IPNs; Apple Academic Press Inc Point: Pleasant, NJ, USA, 2013. [Google Scholar]
- Wei, Y.S.; Wung, B.S.; Lin, Y.C.; Hsieh, C.W. Isolating a cytoprotective compound from Ganoderma tsugae: Effects on induction of Nrf-2-related genes in endothelial cells. Biosci. Biotechnol. Biochem. 2009, 73, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Sun, C.S.; Juan, S.W.; Ho, H.O.; Hu, C.H.; Sheu, M.T. Development of fungal mycelia as skin substitutes: Effects on wound healing and fibroblast. Biomaterials 1999, 20, 61–68. [Google Scholar] [CrossRef]
- Su, C.H.; Sun, C.S.; Juan, S.W.; Hu, C.H.; Ke, W.T.; Sheu, M.T. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997, 18, 1169–1174. [Google Scholar] [CrossRef]
- Chen, C.C.; Cheh, L.W.; Yang, J.C.; Tsai, C.M.; Keh, E.S.; Sheu, M.T. Non-shellfish chitosan from the fruiting body residue of ganoderma tsugae for long-lasting antibacterial guided-tissue regeneration barriers. J. Dent. Sci. 2007, 2, 19–29. [Google Scholar]
- Wang, W.; Bo, S.Q.; Li, S.Q.; Qin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef]
- Sudha, R.; Sukumaran, V.R.; Ranganathan, J.; Bharadwaj, N. Comparative evaluation of the effect of two different concentrations of EDTA at two different pH and time periods on root dentin. J. Conserv. Dent. 2006, 9, 36–42. [Google Scholar] [CrossRef]
- Pearce, E.I. On the dissolution of hydroxyapatite in acid solutions. J. Dent. Res. 1988, 67, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E.J. Environmental Phosphorus Handbook; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Gray, J.A. Kinetics of the dissolution of human dental enamel in acid. J. Dent. Res. 1962, 41, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Zhang, Y.; Carvalho, R.M.; Rueggeberg, F.A.; Russell, C.M. H+-induced tension development in demineralized dentin matrix. J. Dent. Res. 2000, 79, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Hargis, L.G. Analytical Chemistry: Principles and Techniques; Prentice Hall: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Nygaard-Östby, B. Chelation in root canal therapy. Odontol. Tidskr. 1957, 65, 3–11. [Google Scholar]
- Pérez, V.C.; Cárdenas, M.E.M.; Planells, U.S. The possible role of pH changes during EDTA demineralization of teeth. Oral Surg. Oral Med. Oral Pathol. 1989, 68, 220–222. [Google Scholar] [CrossRef]
- Mello, I.; Kammerer, B.A.; Yoshimoto, D.; Macedo, M.C.; Antoniazzi, J.H. Influence of final rinse technique on ability of ethylenediaminetetraacetic acid of removing smear layer. J. Endod. 2010, 36, 512–514. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Saito, K.; Webb, T.D.; Imamura, G.M.; Goodell, G.G. Effect of shortened irrigation times with 17% ethylene diamine tetra-acetic acid on smear layer removal after rotary canal instrumentation. J. Endod. 2008, 34, 1011–1014. [Google Scholar] [CrossRef]
- Calt, S.; Serper, A. Time-dependent effects of EDTA on dentin structures. J. Endod. 2002, 28, 17–19. [Google Scholar] [CrossRef]
- Stewart, G.G.; Kapsimalas, P.; Rappaport, H. EDTA and urea peroxide for root canal preparation. J. Am. Dent. Assoc. 1969, 78, 335–338. [Google Scholar] [CrossRef]
- Kozulic, B. Looking at bands from another side. Anal. Biochem. 1994, 216, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F. Chitin Chemistry; Macmillan: London, UK, 1992. [Google Scholar]
- Qin, Y. The chelating properties of chitosan fibers. J. Appl. Polym. Sci. 1993, 49, 727–731. [Google Scholar] [CrossRef]
- Silva, P.V.; Guedes, D.F.; Pecora, J.D.; da Cruz-Filho, A.M. Time-dependent effects of chitosan on dentin structures. Braz. Dent. J. 2012, 23, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.V.; Guedes, D.F.; Nakadi, F.V.; Pecora, J.D.; Cruz-Filho, A.M. Chitosan: A new solution for removal of smear layer after root canal instrumentation. Int. Endod. J. 2013, 46, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.M.P. Handbook of Naturally Occurring Compounds with Antioxidant Activity in Plants; Nova Science: New York, NY, USA, 2006. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-T.; Teng, N.-C.; Wang, H.-H.; Hsieh, S.-C.; Yang, J.-C. Wasted Ganoderma tsugae Derived Chitosans for Smear Layer Removal in Endodontic Treatment. Polymers 2019, 11, 1795. https://doi.org/10.3390/polym11111795
Huang S-T, Teng N-C, Wang H-H, Hsieh S-C, Yang J-C. Wasted Ganoderma tsugae Derived Chitosans for Smear Layer Removal in Endodontic Treatment. Polymers. 2019; 11(11):1795. https://doi.org/10.3390/polym11111795
Chicago/Turabian StyleHuang, Sheng-Tung, Nai-Chia Teng, Hsin-Hui Wang, Sung-Chih Hsieh, and Jen-Chang Yang. 2019. "Wasted Ganoderma tsugae Derived Chitosans for Smear Layer Removal in Endodontic Treatment" Polymers 11, no. 11: 1795. https://doi.org/10.3390/polym11111795