Photothermal Polymer Nanocomposites of Tungsten Bronze Nanorods with Enhanced Tensile Elongation at Low Filler Contents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TBN Synthesis
2.3. Preparation of TBNR/EPDM Nanocomposite Films
2.4. Characterization
2.5. Photothermal and Mechanical Analysis
3. Results
3.1. Structures, Morphologies, and Compositions of TBNs
3.2. Na0.33WO3 TBNRs with OA Coating
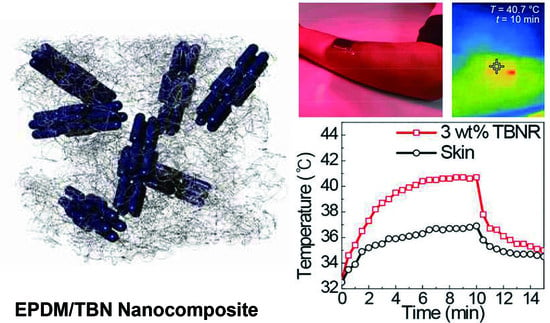
3.3. EPDM Nanocomposite Films with TBNs and Their Photothermal Properties
3.4. Phase Transition and Mechanical Behaviors of Nanocomposite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alkilany, A.M.; Thomson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, D.E.; Kim, E.J.; Ahrberg, C.D.; Chung, B.G. Functional graphene oxide-based nanosheets for photothermal therapy. Macromol. Res. 2018, 26, 557–565. [Google Scholar] [CrossRef]
- Cui, L.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes. WIREs Nanomed. Nanobio. 2017, 9, e1418. [Google Scholar] [CrossRef]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat. Nanotech. 2014, 9, 233–239. [Google Scholar] [CrossRef]
- Yoon, J.; Kwag, J.; Shin, T.J.; Park, J.; Lee, Y.M.; Lee, Y.; Park, J.; Heo, J.; Joo, C.; Park, T.J.; et al. Nanoparticles of conjugated polymers prepared from phase-separated films of phospholipids and polymers for biomedical applications. Adv. Mater. 2014, 26, 4559–4564. [Google Scholar] [CrossRef]
- Guo, C.; Yu, H.; Feng, B.; Gao, W.; Yan, M.; Zhang, Z.; Li, Y.; Liu, S. Highly efficient ablation of metastatic breast cancer using ammonium-tungsten-bronze nanocubes as a novel 064 nm-laser-driven photothermal agent. Biomaterials 2015, 52, 407–416. [Google Scholar] [CrossRef]
- Guo, W.; Guo, C.; Zheng, N.; Sun, T.; Liu, S. CsxWO3 nanorods coated with polyelectrolyte multilayers as a multifunctional nanomaterials for bimodal imaging-guided photothermal/photodynamic cancer treatment. Adv. Mater. 2017, 29, 1604157. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, X.; Zhang, X.; Yin, W.; Ruan, L.; Liu, X.; Zhou, L.; Yan, L.; Li, S.; Gu, Z.; et al. Multifunctional RbxWO3 nanorods for simultaneous combined chemo-photothermal therapy and photoacoustic/ct imaging. Small 2014, 10, 4160–4170. [Google Scholar] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of iron oxide nanoparticles in cancer therapy: Amplification of heating efficiency by magnetic hyperthermia and phtothermal bimodal treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Bogart, L.K.; Taylor, A.; Cesbron, Y.; Murray, P.; Levy, R. Photothermal microscopy of the core of dextran-coated iron oxide nanoparticles during cell uptake. ACS Nano 2012, 6, 5961–5971. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.D.; Seo, Y.H.; Lee, D.; Noh, J.; Chae, J.; Kang, E.; Park, J.H.; Shin, T.J.; Kim, S.; Park, J. Ordered Assemblies of Fe3O4 and a donor-asseptor-type conjugated polymer in nanoparticles for enhanced photoacoustic and magnetic effects. Polymer 2019, 161, 205–213. [Google Scholar] [CrossRef]
- Teodorescu, F.; Queniat, G.; Foulon, C.; Lecoeur, M.; Barras, A.; Boulahneche, S.; Medjram, M.S.; Hubert, T.; Abderrahmani, A.; Boukherroub, R.; et al. Transdermal skin patch based on reduced graphene oxide: A new approach for photothermal triggered permeation of ondansetron across porcine skin. J. Control. Release 2017, 245, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haine, A.T.; Koga, Y.; Hashimoto, Y.; Higashi, T.; Motoyama, K.; Arima, H.; Niidome, T. Enhancement of transdermal protein delivery by photothermal effect of gold nanorods coated on polysaccharide-based hydrogel. Eur. J. Pharm. Biopharm. 2017, 119, 91–95. [Google Scholar] [CrossRef]
- Pissuwan, D.; Nose, K.; Kurihara, R.; Kaneko, K.; Tahara, Y.; Kamiya, N.; Goto, M.; Katayama, Y.; Niidome, T. A solid-in-oil dispersion of gold nanorods can enhance transdermal protein delivery and skin vaccination. Small 2011, 7, 215–220. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lin, Z.-W.; Ling, M.-H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano 2016, 10, 93–101. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Heat: A highly efficient skin enhancer for transdermal drug delivery. Front. Bioeng. Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef]
- Gao, C.; Wang, L.; Lin, Y.; Li, J.; Lin, Y.; Li, X.; Feng, S.; Zheng, Y. Droplets manipulated on photothermal organogel surfaces. Adv. Funct. Mater. 2018, 28, 1803072. [Google Scholar] [CrossRef]
- Bjelobrk, N.; Girard, H.-L.; Subramanyam, S.B.; Kwon, H.-M.; Quere, D.; Varanasi, K.K. Thermocapillary motion on lubricant-impregnated surfaces. Phys. Rev. Fluids 2016, 1, 063902. [Google Scholar] [CrossRef]
- Tang, X.; Wang, L. Loss-free photo-manipulation of droplets by pyroelectro-tranpping on superhydrophobic surfaces. ACS Nano 2018, 12, 8994–9004. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bai, S. Modeling and experimental analysis of thermocapillary effect on laser grooved surfaces at high temperature. Appl. Surf. Sci. 2019, 465, 41–47. [Google Scholar] [CrossRef]
- Chala, T.F.; Wu, C.-M.; Chou, M.-H.; Guo, Z.-L. Melt Electrospun reduced tungsten oxide/polylactic acid fiber membranes as a photothermal material for light-driven interfacial water evaporation. ACS Appl. Mater. Interfaces 2018, 10, 28955–28962. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, D. Spectral selective and photothermal nanostructured thin films for energy efficient windows. Appl. Energy 2017, 208, 83–96. [Google Scholar] [CrossRef]
- Jin, Y.; Chang, J.; Shi, Y.; Shi, L.; Hong, S.; Wang, P. A highly flexible and washable nonwoven photothermal cloth for efficient and practical solar steam generation. J. Mater. Chem. A 2018, 6, 7942–7949. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Park, J.; Hyun, W.; Kim, J.; Kim, J.; Lee, Y.B.; Song, C.; Hwang, H.J.; Kim, J.H.; Hyeon, T.; et al. Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano 2015, 9, 6626–6633. [Google Scholar] [CrossRef]
- Lee, W.R.; Pan, T.L.; Wang, P.W.; Zhuo, R.Z.; Huang, C.M.; Fang, J.Y. Erbium: YAG laser enhances transdermal peptide delivery and skin vaccination. J. Control. Release 2008, 128, 200–208. [Google Scholar] [CrossRef]
- Ramadan, S.; Guo, L.; Li, Y.; Yan, B.; Lu, W. Hollow copper sulfide nanoparticle-mediated transdermal drug delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef]
- Teodorescu, F.; Oz, Y.; Queniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, A.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef]
- Schirmer, O.F.; Bair, G.; Brandt, G. Dependence of WO3 electrochromic absorption on crystallinity. J. Electrochem. Soc. 1977, 124, 749–753. [Google Scholar] [CrossRef]
- Lynch, D.W.; Rosei, R.; Weaver, J.H.; Olson, C.G. The optical properties of some alkali metal tungsten bronzes from 0.1 to 38 eV. J. Solid State Chem. 1973, 8, 242–252. [Google Scholar] [CrossRef]
- Green, M.; Hussain, Z. optical properties of dilute hydrogen tungsten bronze thin films. J. Appl. Phys. 1991, 69, 7788–7796. [Google Scholar] [CrossRef]
- Liu, G.; Wang, S.; Nie, Y.; Sun, X.; Zhang, Y.; Tang, Y. Electrostatic-induced synthesis of tungsten bronze nanostructures with excellent photo-to-thermal conversion behavior. J. Mater. Chem. A 2013, 1, 10120–10129. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Sato, T. Effects of crystallization atmospheres on the near-infrared absorption and electroconductive properties of tungsten bronze Type MxWO3 (M = Na, K). J. Am. Ceram. Soc. 2012, 95, 1634–1639. [Google Scholar] [CrossRef]
- Green, M.; Travlos, A. Sodium-tungsten bronze thin films: I. Optical properties of dilute bronzes. Philos. Mag. B 1985, 51, 501–520. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Yu, H.; Liu, S.; Dong, Q.; Goto, T.; Zhang, Z.; Li, Y.; Sato, T. Phtothermal ablation cancer therapy using homogenous CsxWO3 nanorods with broad near-infrared absorption. Nanoscale 2013, 5, 6469–6478. [Google Scholar] [CrossRef]
- Fei, Y.; Fang, W.; Zhong, M.; Jin, J.; Fan, P.; Yang, J.; Fei, J.; Chen, F.; Kuang, T. Morphological structure, rheological behavior, mechanical properties and sound insulation performance of thermoplastic rubber composites reinforced by different inorganic fillers. Polymers 2018, 10, 276. [Google Scholar] [CrossRef]
- Wang, F.; Hong, R. Continuous preparation of structure-controlled carbon nanoparticle via arc plasma and the reinforcement of polymeric composites. Chem. Eng. J. 2017, 328, 1098–1111. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, E.H.; Kim, S.S. The effects of clay platelets orientation achieved via a dry lamination process on the barrier properties of clay polymer nanocomposites. Macromol. Res. 2018, 26, 454–458. [Google Scholar] [CrossRef]
- Kong, E.J.; Yoon, B.Y.; Nam, J.D.; Suhr, J.H. Accelerated aging and lifetime prediction of graphene-reinforced natural rubber composites. Macromol. Res. 2018, 26, 998–1003. [Google Scholar] [CrossRef]
- Gu, Z.; Ma, Y.; Zhai, T.; Gao, B.; Yang, W.; Yao, J. A Simple hydrothermal method for the large-scale synthesis of singl-crystal potassium tungsten bronze nanowires. Chem. Eur. J. 2006, 12, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yin, S.; Zhang, P.; Yan, M.; Adachi, K.; Chonan, T.; Sato, T. Novel synthesis of homogenous CsxWO3 nanorods with excellent NIR shielding properties by a water controlled-release solvothermal process. J. Mater. Chem. 2010, 20, 8227–8229. [Google Scholar] [CrossRef]
- Jahed, M.; Naderi, G.; Ghoreishy, M.H.R. Microstructure, mechanical, and rheological properties of natural rubber/ethylene propylene diene monomer nanocomposites reinforced by multi-wall carbon nanotubes. Polym. Compos. 2018, 39, E745–E753. [Google Scholar] [CrossRef]
- Bhuyan, B.; Srivastava, S.K.; Puravankara, S.; Mittal, V. Megnesium aluminium layered double hydroxide assisted dispersion of multi-walled carbon nanotubes for enhanced reinforcement of ethylene-co-vinyl acetate matrix. Macromol. Res. 2018, 26, 868–871. [Google Scholar] [CrossRef]
- Koerner, H.; Kelley, J.; George, J.; Drummy, L.; Mirau, P.; Bell, N.S.; Hsu, J.W.P.; Vaia, R.A. ZnO nanorod-thermoplastic polyurethane nanocomposites: Morphology and shape memory performance. Macromolecules 2009, 42, 8933–8942. [Google Scholar] [CrossRef]
- Choi, J.; Moon, K.; Kang, I.; Kim, S.; Yoo, P.J.; Oh, K.W.; Park, J. Preparation of quaternary tungsten bronze nanoparticles by a thermal decomposition of ammonium metatungstate with oleylamine. Chem. Eng. J. 2015, 281, 236–242. [Google Scholar] [CrossRef]
- Reis, K.P.; Ramanan, A.; Whittingham, M.S. Synthesis of novel compounds with the pyrochlore and hexagonal tungsten bronze structures. J. Solid State Chem. 1992, 96, 31–47. [Google Scholar] [CrossRef]
- Fait, M.J.G.; Lunk, H.-J.; Feist, M.; Schneider, M.; Dann, J.N.; Frisk, T.A. Thermal decomposition of ammonium paratungstate tetrahydrate under non-reducing conditions: Characterization by thermal analysis, X-ray diffraction and spectroscopic methods. Thermochim. Acta 2008, 469, 12–22. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzan, L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Zheng, Z.; Yan, B.; Zhang, J.; You, Y.; Lim, C.T.; Shen, Z.; Yu, T. Potassium tungsten bronze nanowires: Polarized micro-raman scattering of individual nanowires and electron field emission from nanowire films. Adv. Mater. 2008, 20, 352–356. [Google Scholar] [CrossRef]
- Solis, J.L.; Hoel, A.; Lantto, V.; Granqvist, C.G. Infrared spectroscopy study of electrochromic nanocrystalline tungsten oxide films made by reactive advanced gas deposition. J. Appl. Phys. 2001, 89, 2727–2732. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.-W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Gao, Y.; Li, X.; Wu, Y.; Zhang, L. Molecular dynamics simulations of the structural, mechanical and visco-elastic properties of polymer nanocomposites filled with grafted nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 7196–7207. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Bittolo Bon, S.; Lopez-Manchado, M.A.; Verdejo, R.; Pappalardo, L.; Bolognini, A.; Alvino, A.; Borsini, S.; Berardo, A.; Pugno, N.M. Synergistic effect of graphene nanoplatelets and carbon black in multifunctional EPDM nanocomposites. Compos. Sci. Technol. 2016, 128, 123–130. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Composto, R.J. Functional polymer nanocomposites enhanced by nanorods. Macromolecules 2014, 47, 875–887. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Chiou, C.-S.; Kumar, S.K.; Lin, J.-J.; Sheng, Y.-J.; Tsao, H.-K. Self-assembled superstructures of polymer-grafted nanoparticles: Effects of particle shape and matrix polymer. J. Phys. Chem. C 2011, 115, 5566–5577. [Google Scholar] [CrossRef]
- Horem, M.J.A.; Frischknecht, A.L.; Composto, R.J. Nanorod assembles in polymer films and their dispersion dependent optical properties. ACS Macro Lett. 2012, 1, 115–121. [Google Scholar] [CrossRef]
- Frischknecht, A.L.; Hore, M.J.A.; Ford, J.; Composto, R.J. Dispersion of polymer-grafted nanorods in homopolymer films: Theory and experiment. Macromolecules 2013, 46, 2856–2869. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Zhang, L.; Lin, X.; Li, H.; Shen, X.; Ganesan, V.; Liu, J. Mechanical and viscoelastic properties of polymer-grafted nanorod composites from molecular dynamics simulation. Macromolecules 2018, 51, 2641–2652. [Google Scholar] [CrossRef]







| Sample | DMA | DSC | Tensile | Photothermal Temp. | |||
|---|---|---|---|---|---|---|---|
| E′ (at 20 °C) (MPa) | Tg (tanδ) (°C) | Tg (°C) | Strength (MPa) | Strain (%) | Max. a (°C) | Rate b (°C/min) | |
| Pristine EPDM | 1.76 | −40.4 | −48.3 | 0.59 ± 0.03 | 120 ± 16 | 22.3 | 0.4 |
| 1 wt% TBNR | 2.14 | −40.8 | −47.2 | 0.55 ± 0.11 | 51 ± 6 | 31.9 | 2.0 |
| 2 wt% TBNR | 3.97 | −41.0 | −48.2 | 0.40 ± 0.06 | 61 ± 18 | 39.2 | 3.3 |
| 3 wt% TBNR | 2.52 | −38.8 | −47.3 | 0.61 ± 0.05 | 165 ± 20 | 41.0 | 3.5 |
| 1 wt% TBNP | – | – | −46.9 | 0.63 ± 0.10 | 130 ± 21 | 34.2 | 2.9 |
| 2 wt% TBNP | – | – | −47.8 | 0.60 ± 0.08 | 93 ± 14 | 39.8 | 3.3 |
| 3 wt% TBNP | – | – | −46.5 | 0.89 ± 0.16 | 98 ± 20 | 42.1 | 3.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, B.; Kim, T.; Lee, D.; Shin, T.J.; Oh, K.W.; Park, J. Photothermal Polymer Nanocomposites of Tungsten Bronze Nanorods with Enhanced Tensile Elongation at Low Filler Contents. Polymers 2019, 11, 1740. https://doi.org/10.3390/polym11111740
Jeon B, Kim T, Lee D, Shin TJ, Oh KW, Park J. Photothermal Polymer Nanocomposites of Tungsten Bronze Nanorods with Enhanced Tensile Elongation at Low Filler Contents. Polymers. 2019; 11(11):1740. https://doi.org/10.3390/polym11111740
Chicago/Turabian StyleJeon, Byoungyun, Taehyung Kim, Dabin Lee, Tae Joo Shin, Kyung Wha Oh, and Juhyun Park. 2019. "Photothermal Polymer Nanocomposites of Tungsten Bronze Nanorods with Enhanced Tensile Elongation at Low Filler Contents" Polymers 11, no. 11: 1740. https://doi.org/10.3390/polym11111740