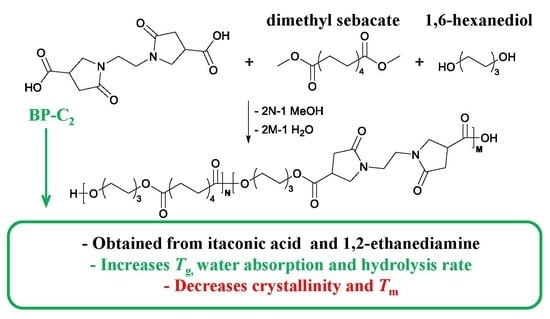
Improving the Hydrolysis Rate of the Renewable Poly(hexamethylene sebacate) Through Copolymerization of a Bis(pyrrolidone)-Based Dicarboxylic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N,N’-Dimethylene-bis(pyrrolidone-4-carboxylic acid)
2.3. Melt Polycondensation Procedures
2.4. Water Absorption and Hydrolysis Experiments
2.5. Enzymatic Depolymerization Experiments
2.6. Characterization Methods
3. Results and Discussion
3.1. Polymer Synthesis
3.2. Thermal Behavior and Crystallinity
3.3. Water Absorption and (Enzymatic) Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, G. Directions for Environmentally Biodegradable Polymer Research. Acc. Chem. Res. 1993, 26, 105–110. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Shah, A.A.; Eguchi, T.; Mayumi, D.; Kato, S.; Shintani, N.; Kamini, N.R.; Nakajima-Kambe, T. Degradation of aliphatic and aliphatic-aromatic co-polyesters by depolymerases from Roseateles depolymerans strain TB-87 and analysis of degradation products by LC-MS. Polym. Degrad. Stab. 2013, 98, 2722–2729. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, B.N. Synthesis and characterization of novel poly(ethylene furanoate-coadipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Suzuki, T.; Takeda, K. Hydrolysis of Polyesters by Rhizopus arrhizus Lipase. Agric. Biol. Chem. 1986, 50, 1323–1325. [Google Scholar] [CrossRef]
- Siotto, M.; Tosin, M.; Innocenti, F.D.; Mezzanotte, V. Mineralization of Monomeric Components of Biodegradable Plastics in Preconditioned and Enriched Sandy Loam Soil Under Laboratory Conditions. Water Air Soil Pollut. 2011, 221, 245–254. [Google Scholar] [CrossRef]
- Siotto, M.; Sezenna, E.; Saponaro, S.; Innocenti, F.D.; Tosin, M.; Bonomo, L.; Mezzanotte, V. Kinetics of monomer biodegradation in soil. J. Environ. Manag. 2012, 93, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Doi, Y. Morphology and enzymatic degradation of poly(l-lactic acid) single crystals. Macromolecules 1998, 31, 2461–2467. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly(l-lactide) 6. Effects of crystallinity on enzymatic hydrolysis of poly(l-lactide) without free amorphous region. Polym. Degrad. Stab. 2001, 71, 415–424. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, H.; Miyauchi, S. Poly(l-lactide): 7. Enzymatic hydrolysis of free and restricted amorphous regions in poly(l-lactide) films with different crystallinities and a fixed crystalline thickness. Polymer 2001, 42, 4463–4467. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Itaconic acid used as a versatile building block for the synthesis of renewable resource-based resins and polyesters for future prospective: A review. Polym. Int. 2017, 66, 1349–1363. [Google Scholar] [CrossRef]
- Qi, P.; Chen, H.-L.; Nguyen, H.T.H.; Lin, C.-C.; Miller, S.A. Synthesis of Biorenewable and Water-Degradable Polylactam Esters from Itaconic Acid. Green Chem. 2016, 18, 4170–4175. [Google Scholar] [CrossRef]
- Noordzij, G.J.; van den Boomen, Y.; Gilbert, C.; van Elk, D.J.P.; Roy, M.; Wilsens, C.H.R.M.; Rastogi, S. The aza-Michael reaction: Towards semi-crystalline polymers from renewable itaconic acid and diamines. Polym. Chem. 2019, 10, 4049–4058. [Google Scholar] [CrossRef]
- Ali, M.A.; Tateyama, S.; Oka, Y.; Kaneko, D.; Okajima, M.K.; Kaneko, T. Syntheses of High-Performance Biopolyamides Derived from Itaconic Acid and Their Environmental Corrosion. Macromolecules 2013, 46, 3719–3725. [Google Scholar] [CrossRef]
- Ali, M.A.; Tateyama, S.; Kaneko, T. Syntheses of rigid-rod but degradable biopolyamides from itaconic acid with aromatic diamines. Polym. Degrad. Stab. 2014, 109, 367–372. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, T.; Xue, X.; He, M.; Xue, J.; Song, M.; Wu, S.; Kang, H.; Zhang, L.; Jia, Q. Synthesis of Fully Bio-Based Polyamides with Tunable Properties by Employing Itaconic Acid. Polymer 2014, 55, 4846–4856. [Google Scholar] [CrossRef]
- Ayadi, F.; Mamzed, S.; Portella, C.; Dole, P. Synthesis of Bis(Pyrrolidone-4-Carboxylic Acid)-Based Polyamides Derived from Renewable Itaconic Acid— Application as a Compatibilizer in Biopolymer Blends. Polym. J. 2013, 45, 766–774. [Google Scholar] [CrossRef]
- Wang, R.; Ren, T.; Bai, Y.; Wang, Y.; Jianfeng, C.; Zhang, L.; Zhao, X. One-pot synthesis of biodegradable and linear poly(ester amide)s based on renewable resources. J. Appl. Polym. Sci. 2016, 43446, 1–6. [Google Scholar] [CrossRef]
- Roy, M.; Noordzij, G.J.; van den Boomen, Y.; Rastogi, S.; Wilsens, C.H.R.M. Renewable (Bis)Pyrrolidone Based Monomers as Components for Thermally Curable and Enzymatically Depolymerizable 2-Oxazoline Thermoset Resins. ACS Sustain. Chem. Eng. 2018, 6, 5053–5066. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Wilsens, C.H.R.M.; Leoné, N.; Rastogi, S. Use of Bis(pyrrolidone)-Based Dicarboxylic Acids in Poly(ester-amide) Based Thermosets: Synthesis, Characterization, and Potential Route for Their Chemical Recycling. ACS Sustain. Chem. Eng. 2019, 7, 8842–8852. [Google Scholar] [CrossRef]
- Krřížek, K.; Ružička, J.; Julinová, M.; Husárová, L.; Houser, J.; Dvoráčková, M.; Jančová, P. N-methyl-2-pyrrolidone-degrading bacteria from activated sludge. Water Sci. Technol. 2015, 71, 776–782. [Google Scholar] [CrossRef]
- Chow, S.T.; Lik Ng, T. The Biodegradation Of N-methyl-2-pyrrolidone in water by sewage bacteria. Water Res. 1983, 17, 117–118. [Google Scholar] [CrossRef]
- Cai, S.; Cai, T.; Liu, S.; Yang, Q.; He, J.; Chen, L.; Hu, J. Biodegradation of N-methylpyrrolidone by Parroccus sp. NMD-4 and its degradation patway. Int. Biodeterior. Biodegrad. 2014, 93, 70–77. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Wullems, N.J.M.; Gubbels, E.; Yao, Y.; Rastogi, S.; Noordover, B.A.J. Synthesis, Kinetics, and Characterization of Bio-Based Thermosets Obtained through Polymerization of a 2,5-Furandicarboxylic Acid-Based Bis(2-Oxazoline) with Sebacic Acid. Polym. Chem. 2015, 6, 2707–2716. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Barlow. J., W.; Paul, D.R. Aliphatic polyester miscibility with polyepichlorohydrin. J. Appl. Polym. Sci. 1984, 29, 1971–1983. [Google Scholar] [CrossRef]
- Armelin, E.; Casas, M.T.; Puigallí, J. Structure of poly(hexamethylene sebacate). Polymer 2001, 42, 5695–5699. [Google Scholar] [CrossRef]








| Polymer | BP-C2 Weighed (mol%) | BP-C2 Observed a (mol%) | Mw (kg/mol) | Ð (-) |
|---|---|---|---|---|
| 0% BP-C2b | 0 | 0 | 37.0 | 7.8 |
| 5% BP-C2 | 5 | 6 | 30.5 | 5.5 |
| 10% BP-C2 | 10 | 10 | 28.0 | 3.2 |
| 25% BP-C2 | 25 | 27 | 28.7 | 3.9 |
| 50% BP-C2 | 50 | 52 | 30.0 | 3.8 |
| 75% BP-C2 | 75 | 76 | 24.8 | 3.7 |
| 100% BP-C2 | 100 | 100 | 18.0 | 3.6 |
| Polymer | Tg (°C) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | X c (%) | T90 (°C) | H2O Uptake (wt %) |
|---|---|---|---|---|---|---|---|---|
| 0% BP-C2 | −60 a | 65.8 | 115.0 | 62.0 | 108.8 | 54 | 343 | 0.32 |
| 5% BP-C2 | - | 61.5 | 113.4 | 47.6 | 111.2 | 52 | 308 | n.d. b |
| 10% BP-C2 | - | 57.9 | 95.6 | 43.7 | 94.2 | 49 | 306 | 1.6 |
| 25% BP-C2 | −42.3 | 49.2 | 67.7 | 27.7 | 63.8 | 38 | 358 | 4.8 |
| 50% BP-C2 | −28.5 | 37.0 | 0.6 | - | - | 8 | 347 | 12.2 |
| 75% BP-C2 | −5.3 | - | - | - | - | - | 351 | 12.4 |
| 100% BP-C2 | 5.7 | - | - | - | - | - | 352 | >15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noordzij, G.J.; Roy, M.; Bos, N.; Reinartz, V.; Wilsens, C.H.R.M. Improving the Hydrolysis Rate of the Renewable Poly(hexamethylene sebacate) Through Copolymerization of a Bis(pyrrolidone)-Based Dicarboxylic Acid. Polymers 2019, 11, 1654. https://doi.org/10.3390/polym11101654
Noordzij GJ, Roy M, Bos N, Reinartz V, Wilsens CHRM. Improving the Hydrolysis Rate of the Renewable Poly(hexamethylene sebacate) Through Copolymerization of a Bis(pyrrolidone)-Based Dicarboxylic Acid. Polymers. 2019; 11(10):1654. https://doi.org/10.3390/polym11101654
Chicago/Turabian StyleNoordzij, Geert. J., Manta Roy, Natasja Bos, Vincent Reinartz, and Carolus H.R.M. Wilsens. 2019. "Improving the Hydrolysis Rate of the Renewable Poly(hexamethylene sebacate) Through Copolymerization of a Bis(pyrrolidone)-Based Dicarboxylic Acid" Polymers 11, no. 10: 1654. https://doi.org/10.3390/polym11101654