Linear and Nonlinear Dynamic Behavior of Polymer Micellar Assemblies Connected by Metallo-Supramolecular Interactions
Abstract
:1. Introduction
2. Experiments
3. Results
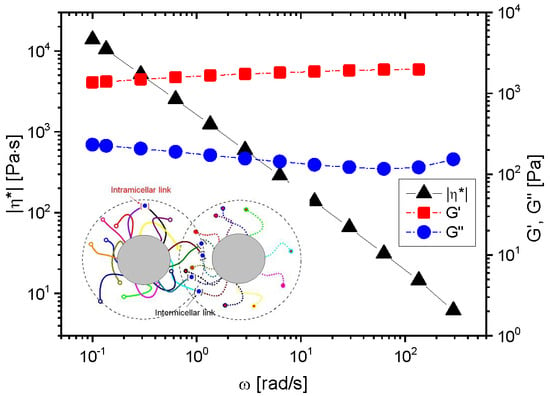
3.1. Structure of the Micellar Network
3.2. Influence of the Type of the Ions and Polymer Concentration
3.3. Entanglement Molecular Weight and Plateau Modulus
3.4. Strain Dependence
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Afifi, H.; da Silva, M.A.; Nouvel, C.; Six, J.L.; Ligoure, C.; Dreiss, C.A. Associative networks of cholesterol-modified dextran with short and long micelles. Soft Matter 2011, 7, 4888–4899. [Google Scholar] [CrossRef]
- Leibler, L.; Rubinstein, M.; Colby, R.H. Dynamics of reversible networks. Macromolecules 1991, 24, 4701–4707. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, P.S.; Baig, C.; Tsolou, G.; Mavrantzas, V.G.; Kroger, M. Quantifying chain reptation in entangled polymer melts: Topological and dynamical mapping of atomistic simulation results onto the tube model. J. Chem. Phys. 2010, 132, 124904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ruymbeke, E.; Orfanou, K.; Kapnistos, M.; Iatrou, H.; Pitsikalis, M.; Hadjichristidis, N.; Lohse, D.J.; Vlassopoulos, D. Entangled dendritic polymers and beyond: Rheology of symmetric cayley-tree polymers and macromolecular self-assemblies. Macromolecules 2007, 40, 5941–5952. [Google Scholar] [CrossRef]
- Ramos, L.; Ligoure, C. Structure of a new type of transient network: Entangled wormlike micelles bridged by telechelic polymers. Macromolecules 2007, 40, 1248–1251. [Google Scholar] [CrossRef]
- Guillet, P.; Mugemana, C.; Stadler, F.J.; Schubert, U.S.; Fustin, C.A.; Bailly, C.; Gohy, J.F. Connecting micelles by metallo-supramolecular interactions: Towards stimuli responsive hierarchical materials. Soft Matter 2009, 5, 3409–3411. [Google Scholar] [CrossRef]
- Lehn, J.M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 2007, 36, 151–160. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [Green Version]
- Lehn, J.M. Supramolecular chemistry: From molecular information towards self-organization and complex matter. Rep. Prog. Phys. 2004, 67, 249–265. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [Green Version]
- Lehn, J.M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Mugemana, C.; Guillet, P.; Fustin, C.A.; Gohy, J.F. Metallo-supramolecular block copolymer micelles: Recent achievements. Soft Matter 2011, 7, 3673–3678. [Google Scholar] [CrossRef]
- Tixier, T.; Tabuteau, H.; Carriere, A.; Ramos, L.; Ligoure, C. Transition from “brittle” to “ductile” rheological behavior by tuning the morphology of self-assembled networks. Soft Matter 2010, 6, 2699–2707. [Google Scholar] [CrossRef]
- Guillet, P.; Fustin, C.A.; Mugemana, C.; Ott, C.; Schubert, U.S.; Gohy, J.F. Tuning block copolymer micelles by metal-ligand interactions. Soft Matter 2008, 4, 2278–2282. [Google Scholar] [CrossRef]
- Brassinne, J.; Gohy, J.F.; Fustin, C.A. Controlling the cross-linking density of supramolecular hydrogels formed by heterotelechelic associating copolymers. Macromolecules 2014, 47, 4514–4524. [Google Scholar] [CrossRef]
- Brassinne, J.; Fustin, C.A.; Gohy, J.F. Control over the assembly and rheology of supramolecular networks via multi-responsive double hydrophilic copolymers. Polym. Chem. 2017, 8, 1527–1539. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent advances in dual temperature responsive block copolymers and their potential as biomedical applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef]
- Vatankhah-Varnoosfaderani, M.; Hashmi, S.; Stadler, F.J.; GhavamiNejad, A. Mussel-inspired 3D networks with stiff-irreversible or soft-reversible characteristics—It’s all a matter of solvent. Polym. Test. 2017, 62, 96–101. [Google Scholar] [CrossRef]
- He, L.; Ran, X.; Li, J.X.; Gao, Q.Q.; Kuang, Y.M.; Guo, L.J. A highly transparent and autonomic self-healing organogel from solvent regulation based on hydrazide derivatives. J. Mater. Chem. A 2018, 6, 16600–16609. [Google Scholar] [CrossRef]
- Yoshida, T.; Taribagil, R.; Hillmyer, M.A.; Lodge, T.P. Viscoelastic synergy in aqueous mixtures of wormlike micelles and model amphiphilic triblock copolymers. Macromolecules 2007, 40, 1615–1623. [Google Scholar] [CrossRef]
- Lodge, T.P.; Taribagil, R.; Yoshida, T.; Hillmyer, M.A. SANS evidence for the cross-linking of wormlike micelles by a model hydrophobically modified polymer. Macromolecules 2007, 40, 4728–4731. [Google Scholar] [CrossRef]
- Vatankhah-Varnoosfaderani, M.; Hashmi, S.; GhavamiNejad, A.; Stadler, F.J. Rapid self-healing and triple stimuli responsiveness of a supramolecular polymer gel based on boron–catechol interactions in a novel water-soluble mussel-inspired copolymer. Polym. Chem. 2014, 5, 512–523. [Google Scholar] [CrossRef]
- Vatankhah-Varnoosfaderani, M.; Ghavaminejad, A.; Hashmi, S.; Stadler, F.J. Mussel-inspired pH-triggered reversible foamed multi-responsive gel—The surprising effect of water. Chem. Commun. 2013, 49, 4685–4687. [Google Scholar] [CrossRef]
- Vatankhah-Varnoosfaderani, M.; GhavamiNejad, A.; Hashmi, S.; Stadler, F.J. Hydrogen bonding in aprotic solvents, a new strategy for gelation of bioinspired catecholic copolymers with N-isopropylamide. Macromol. Rapid Commun. 2015, 36, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Stadler, F.J.; Hashmi, S.; GhavamiNejad, A.; Vatankhah Varnoosfaderani, M. Rheology of dopamine containing polymers. Ann. Trans. Nord. Rheol. Soc. 2017, 25, 115–120. [Google Scholar]
- GhavamiNejad, A.; Sasikala, A.R.K.; Unnithan, A.R.; Thomas, R.G.; Jeong, Y.Y.; Vatankhah-Varnoosfaderani, M.; Stadler, F.J.; Park, C.H.; Kim, C.S. Mussel-Inspired electrospun smart magnetic nanofibers for hyperthermic chemotherapy. Adv. Funct. Mater. 2015, 25, 2867–2875. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Hashmi, S.; Vatankhah-Varnoosfaderani, M.; Stadler, F.J. Effect of H2O and reduced graphene oxide on the structure and rheology of self-healing, stimuli responsive catecholic gels. Rheol. Acta 2016, 55, 163–176. [Google Scholar] [CrossRef]
- Kawamoto, K.; Grindy, S.C.; Liu, J.; Holten-Andersen, N.; Johnson, J.A. Dual role for 1,2,4,5-tetrazines in polymer networks: Combining Diels-Alder reactions and metal coordination to generate functional supramolecular gels. ACS Macro Lett. 2015, 4, 458–461. [Google Scholar] [CrossRef]
- Golkaram, M.; Fodor, C.; van Ruymbeke, E.; Loos, K. Linear viscoelasticity of weakly hydrogen-bonded polymers near and below the sol-gel transition. Macromolecules 2018, 51, 4910–4916. [Google Scholar] [CrossRef]
- Weisstein, E.W. Sphere-Sphere Intersection. Available online: http://mathworld.wolfram.com/Sphere-SphereIntersection.html (accessed on 17 September 2019).
- Stadler, F.J. Quantifying primary loops in polymer gels by linear viscoelasticity. Proc. Natl. Acad. Sci. USA 2013, 110, E1972. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Woo, J.; Cok, A.M.; Wang, M.; Olsen, B.D.; Johnson, J.A. Counting primary loops in polymer gels. Proc. Natl. Acad. Sci. USA 2012, 109, 19119–19124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Du, B.; Stadler, F.J. A novel approach to analyze the rheological properties of hydrogels with network structure simulation. J. Polym. Res. 2018, 25, 4. [Google Scholar] [CrossRef]
- Daoud, M.; Cotton, J.P. Star shaped polymers: A model for the conformation and its concentration dependence. J. Phys. 1982, 43, 531–538. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Pakula, T.; Fytas, G.; Roovers, J.; Karatasos, K.; Hadjichristidis, N. Ordering and viscoelastic relaxation in multiarm star polymer melts. Polymer 1997, 39, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Holyer, R.H.; Hubbard, C.D.; Kettle, S.F.A.; Wilkins, R.G. The Kinetics of Replacement Reactions of Complexes of the Transition Metals with 1,10-Phenanthroline and 2,2′-Bipyridine. Inorg. Chem. 1965, 4, 929–935. [Google Scholar] [CrossRef]
- Dobrawa, R.; Wurthner, F. Metallosupramolecular approach toward functional coordination polymers. J. Polym. Sci. Polym. Chem. 2005, 43, 4981–4995. [Google Scholar] [CrossRef]
- Osaki, K.; Nishimura, Y.; Kurata, M. Viscoelastic Properties of Semidilute Polystyrene Solutions. Macromolecules 1985, 18, 1153–1157. [Google Scholar] [CrossRef]
- Fetters, L.J.; Lohse, D.J.; Colby, R.H. Chain dimensions and entanglement spacings. In Physical Properties of Polymers, 2nd ed.; Mark, J.E., Ed.; Springer: Heidelberg, Germany, 2007. [Google Scholar]
- Tong, J.D.; Leclere, P.; Doneux, C.; Bredas, J.L.; Lazzaroni, R.; Jerome, R. Morphology and mechanical properties of poly(methylmethacrylate)-b-poly(alkylacrylate)-b-poly(methylmethacrylate). Polymer 2001, 42, 3503–3514. [Google Scholar] [CrossRef]
- Zeng, F.; Han, Y.; Yan, Z.-C.; Liu, C.-Y.; Chen, C.-F. Supramolecular polymer gel with multi stimuli responsive, self-healing and erasable properties generated by host–guest interactions. Polymer 2013, 54, 6929–6935. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Liu, Z.-X.; Yan, Z.-C.; He, Y.-M.; Liu, C.-Y.; Fan, Q.-H. N-Boc-Protected 1,2-Diphenylethylenediamine-Based Dendritic Organogels with Multiple-Stimulus-Responsive Properties. Chem. Asian J. 2013, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-X.; Feng, Y.; Zhao, Z.-Y.; Yan, Z.-C.; He, Y.-M.; Luo, X.-J.; Liu, C.-Y.; Fan, Q.-H. A New Class of Dendritic Metallogels with Multiple Stimuli-Responsiveness and as Templates for the In Situ Synthesis of Silver Nanoparticles. Chem. Eur. J. 2014, 20, 533–541. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Feng, Y.; Yan, Z.-C.; He, Y.-M.; Liu, C.-Y.; Fan, Q.-H. Multistimuli Responsive Dendritic Organogels Based on Azobenzene-Containing Poly(aryl ether) Dendron. Chem. Mater. 2012, 24, 3751–3757. [Google Scholar] [CrossRef]
- Sim, H.G.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear behavior of complex fluids investigated by a network model: A guideline for classification. J. Non-Newton. Fluid 2003, 112, 237–250. [Google Scholar] [CrossRef]
- Berret, J.F.; Calvet, D.; Collet, A.; Viguier, M. Fluorocarbon associative polymers. Curr. Opin. Colloid Interface Sci. 2003, 8, 206–306. [Google Scholar] [CrossRef]
- Tirtaatmadja, V.; Tam, K.C.; Jenkins, R.D. Superposition of oscillations on steady shear flow as a technique for investigating the structure of associative polymers. Macromolecules 1997, 30, 1426–1433. [Google Scholar] [CrossRef]
- Annable, T.; Buscall, R.; Ettelaie, R.; Whittlestone, D. The rheology of solutions of associating polymers—Comparison of experimental behavior with transient network theory. J. Rheol. 1993, 37, 695–726. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S.F. Viscoelastic properties of physically cross-linked networks. 1. Nonlinear stationary viscoelasticity. J. Non-Newton. Fluid 1992, 43, 247–271. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S.F. Viscoelastic properties of physically cross-linked networks. 2. Dynamic mechanical moduli. J. Non-Newton. Fluid 1992, 43, 273–288. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S.F. Viscoelastic properties of physically cross-linked networks. 3. Time-dependent phenomena. J. Non-Newton. Fluid 1992, 43, 289–309. [Google Scholar] [CrossRef]
- Yziquel, F.; Carreau, P.J.; Moan, M.; Tanguy, P.A. Rheological modeling of concentrated colloidal suspensions. J. Non-Newton. Fluid 1999, 86, 133–155. [Google Scholar] [CrossRef]
- Hamley, I.W.; Castelletto, V. Small-angle scattering of block copolymers in the melt, solution and crystal states. Prog. Polym. Sci. 2004, 29, 909–948. [Google Scholar]
- Hamley, I.W.; Pople, J.A.; Booth, C.; Derici, L.; Imperor-Clerc, M.; Davidson, P. Shear-induced orientation of the body-centered-cubic phase in a diblock copolymer gel. Phys. Rev. E 1998, 58, 7620–7628. [Google Scholar] [CrossRef]
- Pople, J.A.; Hamley, I.W.; Diakun, G.P. An integrated Couette system for in situ shearing of polymer and surfactant solutions and gels with simultaneous small angle x-ray scattering. Rev. Sci. Instrum. 1998, 69, 3015–3021. [Google Scholar] [CrossRef]
- Sing, M.K.; Glassman, M.J.; Vronay-Ruggles, X.T.; Burghardt, W.R.; Olsen, B.D. Structure and rheology of dual-associative protein hydrogels under nonlinear shear flow. Soft Matter 2017, 13, 8511–8524. [Google Scholar] [CrossRef]
- Caputo, F.E.; Ugaz, V.M.; Burghardt, W.R.; Berret, J.-F. Transient 1–2 plane small-angle x-ray scattering measurements of micellar orientation in aligning and tumbling nematic surfactant solutions. J. Rheol. 2002, 46, 927–946. [Google Scholar] [CrossRef]
- Caputo, F.E.; Burghardt, W.R.; Krishnan, K.; Bates, F.S.; Lodge, T.P. Time-resolved small-angle X-ray scattering measurements of a polymer bicontinuous microemulsion structure factor under shear. Phys. Rev. E 2002, 66, 041401. [Google Scholar] [CrossRef]
- Martin, H.P.; Brooks, N.J.; Seddon, J.M.; Luckham, P.F.; Terrill, N.J.; Kowalski, A.J.; Cabral, J.T. Microfluidic processing of concentrated surfactant mixtures: Online SAXS, microscopy and rheology. Soft Matter 2016, 12, 1750–1758. [Google Scholar] [CrossRef]
- Kim, J.M.; Eberle, A.P.R.; Gurnon, A.K.; Porcar, L.; Wagner, N.J. The microstructure and rheology of a model, thixotropic nanoparticle gel under steady shear and large amplitude oscillatory shear (LAOS). J. Rheol. 2014, 58, 1301–1328. [Google Scholar] [CrossRef]
- Calabrese, M.A.; Rogers, S.A.; Murphy, R.P.; Wagner, N.J. The rheology and microstructure of branched micelles under shear. J. Rheol. 2015, 59, 1299–1328. [Google Scholar] [CrossRef]
- Rogers, S.; Kohlbrecher, J.; Lettinga, M.P. The molecular origin of stress generation in worm-like micelles, using a rheo-SANS LAOS approach. Soft Matter 2012, 8, 7831–7839. [Google Scholar] [CrossRef] [Green Version]
- Lonetti, B.; Kohlbrecher, J.; Willner, L.; Dhont, J.K.G.; Lettinga, M.P. Dynamic response of block copolymer wormlike micelles to shear flow. J. Phys. Condens. Matter 2008, 20, 404207. [Google Scholar] [CrossRef]
- Lopez-Barron, C.R.; Wagner, N.J.; Porcar, L. Layering, melting, and recrystallization of a close-packed micellar crystal under steady and large-amplitude oscillatory shear flows. J. Rheol. 2015, 59, 793–820. [Google Scholar] [CrossRef]
- Westermeier, F.; Pennicard, D.; Hirsemann, H.; Wagner, U.H.; Rau, C.; Graafsma, H.; Schall, P.; Paul Lettinga, M.; Struth, B. Connecting structure, dynamics and viscosity in sheared soft colloidal liquids: A medley of anisotropic fluctuations. Soft Matter 2016, 12, 171–180. [Google Scholar] [CrossRef]












| 12 w/v% | 15 w/v% | |
|---|---|---|
| ϕ [-] | 0.156 | 0.193 |
| n [mol/m3] | 0.50 | 0.65 |
| G0,theor (Equation (7)) [Pa] a | 1200 | 1600 |
| G0,exp.(experimental, Fe2+-containing samples at 10 rad/s) [Pa] | 450 | 1900 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.-C.; Stadler, F.J.; Guillet, P.; Mugemana, C.; Fustin, C.-A.; Gohy, J.-F.; Bailly, C. Linear and Nonlinear Dynamic Behavior of Polymer Micellar Assemblies Connected by Metallo-Supramolecular Interactions. Polymers 2019, 11, 1532. https://doi.org/10.3390/polym11101532
Yan Z-C, Stadler FJ, Guillet P, Mugemana C, Fustin C-A, Gohy J-F, Bailly C. Linear and Nonlinear Dynamic Behavior of Polymer Micellar Assemblies Connected by Metallo-Supramolecular Interactions. Polymers. 2019; 11(10):1532. https://doi.org/10.3390/polym11101532
Chicago/Turabian StyleYan, Zhi-Chao, Florian J. Stadler, Pierre Guillet, Clément Mugemana, Charles-André Fustin, Jean-François Gohy, and Christian Bailly. 2019. "Linear and Nonlinear Dynamic Behavior of Polymer Micellar Assemblies Connected by Metallo-Supramolecular Interactions" Polymers 11, no. 10: 1532. https://doi.org/10.3390/polym11101532