A Statistical Analysis on the Effect of Antioxidants on the Thermal-Oxidative Stability of Commercial Mass- and Emulsion-Polymerized ABS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Analysis
2.2.1. Simultaneous Thermal Analysis (STA)
2.2.2. Processing
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Oxidation Onset Temperature (OOT)
2.2.5. Yellowing Index (YI)
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Simultaneous Thermal Analysis (STA)
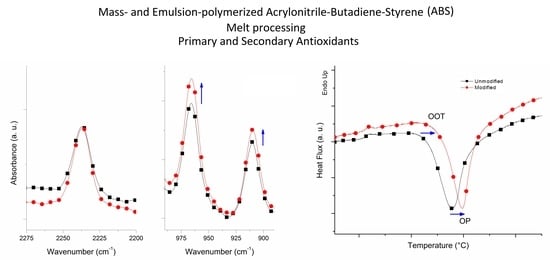
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Oxidation Onset Temperature (OOT)
3.4. Yellowing Index (YI)
3.5. Summary of the ANOVA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor—2017; United Nations University (UNU): Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Vienna, Austria, 2017. [Google Scholar]
- Delva, L.; Hubo, S.; Cardon, L.; Ragaert, K. On the role of flame retardants in mechanical recycling of solid plastic waste. Waste Manag. 2018, 82, 198–206. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE). Off. J. Eur. Union 2012, L197, 38–71. [Google Scholar]
- Wäger, P.A.; Hischier, R. Life cycle assessment of post-consumer plastics production from waste electrical and electronic equipment (WEEE) treatment residues in a Central European plastics recycling plant. Sci. Total Environ. 2015, 529, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Guo, J.; Zhang, W.; Summers, P.A.; Hall, P. From waste plastics to industrial raw materials: A life cycle assessment of mechanical plastic recycling practice based on a real-world case study. Sci. Total Environ. 2017, 601–602, 1192–1207. [Google Scholar] [CrossRef] [PubMed]
- Stenvall, E.; Tostar, S.; Boldizar, A.; Foreman, M.R.S.; Möller, K. An analysis of the composition and metal contamination of plastics from waste electrical and electronic equipment (WEEE). Waste Manag. 2013, 33, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Botané, P.; Wavrer, P.; Froelich, D. Characterizing plastics originating from WEEE: A case study in France. Miner. Eng. 2015, 76, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Freegard, K.; Claes, M. Compositional Analysis of Kerbside Collected Small WEEE; Axion Recycling Ltd.: Banbury, UK, 2009. [Google Scholar]
- Ragaert, K.; Delva, L.; Geem, K.V. Mechanical and chemical recycling of solid plastic wast. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Domingo, F.J.V. Analytical Strategies for the Quality Assesment of Recycled High Impact Polystyrene (HIPS); Universidad Politécnica de Valencia: Valencia, Spain, 2008. [Google Scholar]
- Adeniyi, J.B. Clarification and discussion of chemical transformations involved in thermal and photo-oxidative degradation of ABS. Eur. Polym. J. 1984, 20, 291–299. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Degradation of recycled high-impact polystyrene. Simulation by reprocessing and thermo-oxidation. Polym. Degrad. Stab. 2006, 91, 2163–2217. [Google Scholar] [CrossRef]
- Shimada, J.; Kabuki, K. The mechanism of oxidative degradation of ABS resin. Part I. The mechanism of thermooxidative degradation. J. Appl. Polym. Sci. 1968, 12, 655–669. [Google Scholar] [CrossRef]
- Zweifel, H. Stabilization of Polymeric Materials; Springer: Berlin, Germany, 1998. [Google Scholar]
- Adeniyi, J.B.; Kolawole, E.G. Thermal and photo-degradation of unstabilized ABS. Eur. Polym. J. 1984, 20, 43–47. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Benedetto, G.D. Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Thürmer, A.; Al-Malaika, S.; Pospísil, J. Plastics Additives—An A-Z Reference; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Meier, H.; Dubs, P.; Künzi, H.; Martin, R.; Knobloch, G.; Bertterman, H.; Thuet, B.; Borer, A.; Kolczak, U.; Rist, G. Some aspects of a new class of sulfur containing phenolic antioxidants. Polym. Degrad. Stab. 1995, 49, 1–9. [Google Scholar] [CrossRef]
- Paoli, M.A.D.; Schultz, G.W.; Furlan, L.T. Commercial antioxidants as photostabilizers for butadiene rubber. J. Appl. Polym. Sci. 1984, 29, 2493–2500. [Google Scholar] [CrossRef]
- Földes, E.; Lohmeijer, J. Relationship between chemical structure and performance of primary antioxidants in PBD. Polym. Degrad. Stab. 1999, 66, 31–39. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Ho, T.-C.; Chen, J.-R.; Kao, C.-S. Study on Exothermic Oxidation of Acrylonitrile-butadienestyrene (ABS) Resin Powder with Application to ABS Processing Safety. Polymers 2010, 2, 174–187. [Google Scholar] [CrossRef]
- Motyakin, M.; Schlick, S. ESR imaging and FTIR study of thermally treated poly(acrylonitrile-butadiene-styrene) (ABS) containing a hindered amine stabilizer: Effect of polymer morphology, and butadiene and stabilizer content. Polym. Degrad. Stab. 2006, 91, 1462–1470. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.; Reyniers, M.F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Moon, B.; Lee, J.; Park, S.; Seok, C.-S. Study on the Aging Behavior of Natural Rubber/Butadiene Rubber (NR/BR) Blends Using a Parallel Spring Model. Polymers 2018, 10, 658. [Google Scholar] [CrossRef]
- Ineos-Styrolution. Technical Datasheet—Terluran GP-22; Ineos-Styrolution: Antwerp, Belgium, 2016; p. 3. [Google Scholar]
- Trinseo. Technical Information—Magnum 3453; Trinseo: Terneuzen, The Netherlands, 2017; p. 3. [Google Scholar]
- Santos, R.M.; Botelho, G.L.; Machado, A.V. Artificial and natural weathering of ABS. J. Appl. Polym. Sci. 2010, 116, 2005–2014. [Google Scholar] [CrossRef]
- Bai, X.; Isaac, D.H.; Smith, K. Reprocessing Acrylonitrile–Butadiene–Styrene Plastics: Structure–Property Relationships. Polym. Eng. Sci. 2007, 47, 120–130. [Google Scholar] [CrossRef]
- ASTM. E 2009—Standard Test Methods for Oxidation Onset Temperature of Hydrocarbons by Differential Scanning Calorimetry; ASTM International: West Conshohocken, PA, USA, 2014; p. 5. [Google Scholar]
- ASTM. E 313—Standard Practice for Calculating Yellowness and Whiteness Indices from Instrumentally Measured Color Coordinates; ASTM International: West Conshohocken, PA, USA, 2015; p. 6. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- BASF. Technical Datasheet—Irgafos 168, June 2016 ed.; BASF: Charlotte, NC, USA, 2016; p. 3. [Google Scholar]
- BASF. Technical Datasheet—Irganox 245; BASF: Charlotte, NC, USA, 2015; p. 3. [Google Scholar]
- BASF. Technical Datasheet—Irganox 1076; Basf Corporation: Charlotte, NC, USA, 2015; p. 3. [Google Scholar]
- Gesner, B.D. Analysis of acrylonitrile-butadiene-styrene (ABS) plastics by infrared spectroscopy. In Developments in Applied Spectroscopy; Grove, E.L., Perkins, A.J., Eds.; Plenum Press: New York, NY, USA, 1970; Volume 7B. [Google Scholar]
- Piton, M.; Rivaton, A. Photo-oxidation of ABS at long wavelengths (λ > 300 nm). Polym. Degrad. Stab. 1997, 55, 147–157. [Google Scholar] [CrossRef]
- Giaconi, G.F.; Castellani, L.; Maestrini, C.; Ricco, T. Development of toughness in ABS resins. Polymer 1998, 39, 6315–6324. [Google Scholar] [CrossRef]







| Experiment | Sample Code | Irganox 1076 (m%) | Irganox 245 (m%) | Irgafos 168 (m%) | Type of ABS |
|---|---|---|---|---|---|
| 1 | 0/0/0/M | 0.0 | 0.0 | 0.0 | Mass |
| 2 | 0.2/0/0/M | 0.2 | 0.0 | 0.0 | Mass |
| 3 | 0/0.2/0/M | 0.0 | 0.2 | 0.0 | Mass |
| 4 | 0.2/0.2/0/M | 0.2 | 0.2 | 0.0 | Mass |
| 5 | 0/0/0.2/M | 0.0 | 0.0 | 0.2 | Mass |
| 6 | 0.2/0/0.2/M | 0.2 | 0.0 | 0.2 | Mass |
| 7 | 0/0.2/0.2/M | 0.0 | 0.2 | 0.2 | Mass |
| 8 | 0.2/0.2/0.2/M | 0.2 | 0.2 | 0.2 | Mass |
| 9 | 0/0/0/E | 0.0 | 0.0 | 0.0 | Emulsion |
| 10 | 0.2/0/0/E | 0.2 | 0.0 | 0.0 | Emulsion |
| 11 | 0/0.2/0/E | 0.0 | 0.2 | 0.0 | Emulsion |
| 12 | 0.2/0.2/0/E | 0.2 | 0.2 | 0.0 | Emulsion |
| 13 | 0/0/0.2/E | 0.0 | 0.0 | 0.2 | Emulsion |
| 14 | 0.2/0/0.2/E | 0.2 | 0.0 | 0.2 | Emulsion |
| 15 | 0/0.2/0.2/E | 0.0 | 0.2 | 0.2 | Emulsion |
| 16 | 0.2/0.2/0.2/E | 0.2 | 0.2 | 0.2 | Emulsion |
| Property | Main Factor (Code) | Significant Interaction(s) | |||
|---|---|---|---|---|---|
| Irganox 1076a (‘A’) | Irganox 245a (‘B’) | Irgafos 168b (‘C’) | Type of ABS (‘D’) | ||
| R1 | I | I | I | S (+1) | - |
| R2 | I | I | I | S (+1) | - |
| R3 | I | S (+1) | I | S (+1) | AC; BD |
| R4 | I | S (+1) | I | S (+1) | AD; BD |
| OOT | S (+1) | S (+1) | I | S (−1) | AB |
| OP | S (+1) | S (+1) | S (+1) | S (−1) | AB; ABD |
| YI | I | I | I | S (+1) | AB |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorio, R.; D’hooge, D.R.; Ragaert, K.; Cardon, L. A Statistical Analysis on the Effect of Antioxidants on the Thermal-Oxidative Stability of Commercial Mass- and Emulsion-Polymerized ABS. Polymers 2019, 11, 25. https://doi.org/10.3390/polym11010025
Fiorio R, D’hooge DR, Ragaert K, Cardon L. A Statistical Analysis on the Effect of Antioxidants on the Thermal-Oxidative Stability of Commercial Mass- and Emulsion-Polymerized ABS. Polymers. 2019; 11(1):25. https://doi.org/10.3390/polym11010025
Chicago/Turabian StyleFiorio, Rudinei, Dagmar R. D’hooge, Kim Ragaert, and Ludwig Cardon. 2019. "A Statistical Analysis on the Effect of Antioxidants on the Thermal-Oxidative Stability of Commercial Mass- and Emulsion-Polymerized ABS" Polymers 11, no. 1: 25. https://doi.org/10.3390/polym11010025