Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
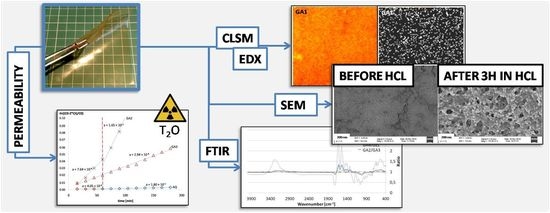
2.3. Scanning Electron Microscopy (SEM)
2.4. Energy-Dispersive X-ray Spectroscopy (EDX)
2.5. Confocal Laser Scanning Microscopy (CLSM)
2.6. Attenuated Total Reflection-Fourier Transform Infra-Red (ATR-FTIR) Spectroscopy
2.7. Swelling and Fraction Soluble in HCl
2.8. Permeability Study
3. Results and Discussion
3.1. Microscopic Structure
3.2. Permeability
3.3. ATR-FTIR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Gelatin microspheres: Influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 1996, 17, 2009–2020. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Flaker, C.H.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.A. Gelatin-based nanocomposite films: A study on montmorillonite dispersion methods and concentration. J. Food Eng. 2015, 167, 65–70. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Devi, N.; Kakati, D.K. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J. Food Eng. 2013, 117, 193–204. [Google Scholar] [CrossRef]
- Djabourov, M.; Papon, P. Influence of thermal treatments on the structure and stability of gelatin gels. Polymer 1983, 24, 537–542. [Google Scholar] [CrossRef]
- Nur Hanani, Z.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Karim, A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Rabadiya, B.; Rabadiya, P. Review: Capsule shell material from gelatin to non animal origin material. Int. J. Pharm. Res. BioSci. 2013, 2, 42–71. [Google Scholar]
- Cerea, M.; Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Sangalli, M.E. Dry coating of soft gelatin capsules with HPMCAS. Drug Dev. Ind. Pharm. 2008, 34, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Haase, M.M.; Shah, N.H.; Zhang, G.; Infeld, M.H.; Malick, A.W.; Mcginity, J.W. Physical and enteric properties of soft gelatin capsules coated with Eutragit L30 D-55. Int. J. Pharm. 1995, 113, 17–24. [Google Scholar] [CrossRef]
- Fox, S.H.; Paterson, O.L. Enteric gelatin Capsule Shell or Envelope. U.S. Patent 2390088A, 1945. [Google Scholar]
- Bogin, H.H. Enteric Capsule. U.S. Patent 2491475, 1949. [Google Scholar]
- Hassan, E.M.; Fatmi, A.A.; Chidambaram, N. Enteric Composition for the Manufacture of Soft Capsule Wall. U.S. Patent 8685445, 2014. [Google Scholar]
- Teles, H.; Van Duijnhoven, H.; Bayarri, M. Enteric Soft Capsule Compositions. WO Application WO 2015195989A1, 2015. [Google Scholar]
- Maciejewski, B.; Weitschies, W.; Schneider, F.; Sznitowska, M. Gastroresistant gelatin films prepared by addition of cellulose acetate phthalate. Pharmazie 2017, 72, 324–328. [Google Scholar] [PubMed]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar] [PubMed]
- Hjaertstam, J.; Hjertberg, T. Studies of the water permeability and mechanical properties of a film made of an ethyl cellulose-ethanol-water ternary mixture. J. Appl. Polym. Sci. 1999, 74, 2056–2062. [Google Scholar] [CrossRef]
- Andersson, H.; Hjärtstam, J.; Stading, M.; von Corswant, C.; Larsson, A. Effects of molecular weight on permeability and microstructure of mixed ethyl-hydroxypropyl-cellulose films. Eur. J. Pharm. Sci. 2013, 48, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G.; Samyn, C.; Kinget, R. Characterization of colon-specific azo polymers: A study of the swelling propertoes and the permeability of isolated polymer films. Int. J. Pharm. 1994, 111, 127–136. [Google Scholar] [CrossRef]
- Tromp, R.H.; Van de Velde, F.; Van Riel, J.; Paques, M. Confocal scanning light microscopy (CSLM) on mixtures of gelatine and polysaccharides. Food Res. Int. 2001, 34, 931–938. [Google Scholar] [CrossRef]
- Felder, C.B.; Blanco-Prieto, M.J.; Heizmann, J.; Merkle, H.P.; Gander, B. Ultrasonic atomization and subsequent polymer desolvation for peptide and protein microencapsulation into biodegradable polyesters. J. Microencapsul. 2003, 20, 553–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Weiss, G.; Knoch, A.; Laicher, A.; Stanislaus, F.; Daniels, R. Simple coacervation of hydroxypropyl methylcellulose phthalate (HPMCP)I. Temperature and pH dependency of coacervate formation. Int. J. Pharm. 1995, 124, 87–96. [Google Scholar]
- Williams, R.O.; Liu, J. Influence of processing and curing conditions on beads coated with an aqueous dispersion of cellulose acetate phthalate. Eur. J. Pharm. Biopharm. 2000, 49, 243–252. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of fish gelatin films added with gellan and kappa-carrageenan. LWTFood Sci. Technol. 2007, 40, 766–774. [Google Scholar]
- Michon, C.; Cuvelier, G.; Launay, B.; Parker, A. Viscoelastic properties of iota-carrageenan/gelatin mixtures. Carbohydr. Polym. 1996, 31, 161–169. [Google Scholar] [CrossRef]
- Hashim, D.M.; Man, Y.B.C.; Norakasha, R.; Shuhaimi, M.; Salmah, Y.; Syahariza, Z.A. Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chem. 2010, 118, 856–860. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J. Food Eng. 2010, 96, 66–73. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT Food Sci. Technol. 2015, 63, 1–8. [Google Scholar] [CrossRef]







| Symbol | % Aquacoat CPD * | Composition of Dry Film (%) | |||
|---|---|---|---|---|---|
| Gelatin | Aquacoat CPD | Glycerol | ί-Carrageenan | ||
| GA1 | 30% | 48.1 | 20.6 | 31.3 | - |
| GA2 | 25% | 51.5 | 17.2 | 31.3 | - |
| GA3 | 25% | 50.0 | 17.2 | 31.3 | 1.5 |
| GA4 | 10% | 61.8 | 6.9 | 31.3 | - |
| GEL | 0% | 68.7 | - | 31.3 | - |
| AQ | 100% | - | 100.0 | - | - |
| Sample | Content (% m/m) in the Investigated Films | Residue (% of the Initial Mass) * | ||
|---|---|---|---|---|
| Aquacoat CPD Content | Iota-Carrageenan | |||
| Total | CAP | |||
| GA1 | 20.6% | 15.8% | - | 16.4 ± 2.1% |
| GA2 | 17.2% | 13.2% | - | 15.5 ± 1.2% |
| GA3 | 17.2% | 13.2% | 1.5% | 38.3 ± 5.1% |
| GA4 | 6.9% | 5.3% | - | 12.4 ± 2.0% |
| Films | Permeation of Water * | ||||
|---|---|---|---|---|---|
| Sample | Aquacoat % (Based on Total Polymer Content) | Thickness (µm) | Cumulative Amount after 3 h (% of the Total Amount) | Average Permeability (cm2/s) | |
| 15–60 min | 60–180 min | ||||
| GA1 | 30% | 540 ± 17 | 7.6% | 2.65 × 10−6 | 4.02 × 10−6 |
| GA2 | 25% | 509 ± 27 | 10.9% | 3.53 × 10−6 | 5.95 × 10−6 |
| GA3 | 25% (+1.5% carrageenan) | 622 ± 35 | 2.3% | 1.1 × 10−6 | |
| GA4 | 10% | 446 ± 17 | n/a | 8.19 × 10−6 | Disintegration after 100–150 min |
| AQ | 100% | 600 ± 17 | 0.15% | 1.67 × 10−8 | 7.58 × 10−8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewski, B.; Ström, A.; Larsson, A.; Sznitowska, M. Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability. Polymers 2018, 10, 981. https://doi.org/10.3390/polym10090981
Maciejewski B, Ström A, Larsson A, Sznitowska M. Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability. Polymers. 2018; 10(9):981. https://doi.org/10.3390/polym10090981
Chicago/Turabian StyleMaciejewski, Bartosz, Anna Ström, Anette Larsson, and Małgorzata Sznitowska. 2018. "Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability" Polymers 10, no. 9: 981. https://doi.org/10.3390/polym10090981