Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
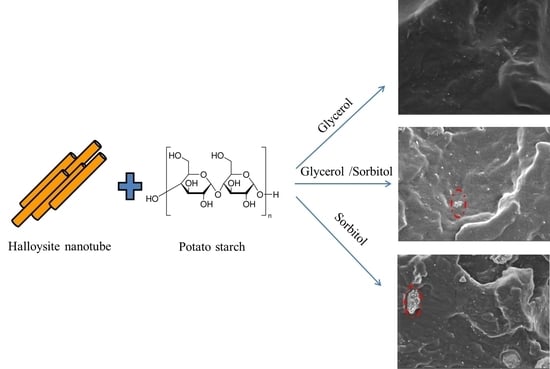
2.2. Preparation of Nanocomposites
2.2.1. Preparation of Plasticized Starch Dry-Blends
2.2.2. Nano-Biocomposites Elaboration
2.3. Characterization Techniques
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Density and Moisture Measurements
2.3.6. Mechanical Properties
3. Results and Discussion
3.1. Morphological Characterization
3.2. Chemical Interactions
3.3. Microstructure and Crystallinity Studies
3.4. Thermal Stability
3.5. Density and Moisture Content
3.6. Uniaxial Tensile Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Xie, F.; Halley, P.J.; Avérous, L. Rheology to understand and optimize processibility, structures and properties of starch polymeric materials. Prog. Polym. Sci. 2012, 37, 595–623. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, W.; Yu, Y.; Zhang, G.; Guo, W. Preparation and structure characterization of linear long-chain dextrin obtained from pullulanase debranching of cassava starch. Starch-Stärke 2015, 67, 884–891. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, W.; Lou, F.; Wang, Y.; Guo, W. Characteristics of starch-based films produced using glycerol and 1-butyl-3-methylimidazolium chloride as combined plasticizers. Starch-Stärke 2017, 69, 160161. [Google Scholar] [CrossRef]
- Averous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-based nano-biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef]
- Glenn, G.M.; Orts, W.; Imam, S.; Chiou, B.-S.; Wood, D.F. Starch plastic packaging and agriculture applications. In Starch Polymers; Halley, P., Avérous, L., Eds.; Elsevier: Amsterdam, The Netherland, 2014; pp. 421–452. ISBN 978-0-444-53730-0. [Google Scholar]
- Dean, K.M.; Do, M.D.; Petinakis, E.; Yu, L. Key interactions in biodegradable thermoplastic starch/poly(vinyl alcohol)/montmorillonite micro- and nanocomposites. Compos. Sci. Technol. 2008, 68, 1453–1462. [Google Scholar] [CrossRef]
- Sarazin, P.; Li, G.; Orts, W.J.; Favis, B.D. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch. Polymer 2008, 49, 599–609. [Google Scholar] [CrossRef]
- Chang, P.R.; Wu, D.; Anderson, D.P.; Ma, X. Nanocomposites based on plasticized starch and rectorite clay: Structure and properties. Carbohydr. Polym. 2012, 89, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in hydroxyapatite–starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Thakur, V.K. Recent advances in starch–clay nanocomposites. Polymers 2018, 10, 467. [Google Scholar] [CrossRef]
- Avérous, L.; Pollet, E. Green nano-biocomposites. In Environmental Silicate Nano-Biocomposites; Avérous, L., Pollet, E., Eds.; Springer: London, UK, 2012; pp. 1–11. ISBN 978-1-4471-4101-3. [Google Scholar]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. New approach to elaborate exfoliated starch-based nanobiocomposites. Biomacromolecules 2008, 9, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Evans, J.R.G. Thermoplastic starch–clay nanocomposites and their characteristics. Carbohydr. Polym. 2005, 61, 455–463. [Google Scholar] [CrossRef]
- Ismail, H.; Pasbakhsh, P.; Fauzi, M.N.A.; Abu Bakar, A. Morphological, thermal and tensile properties of halloysite nanotubes filled ethylene propylene diene monomer (EPDM) nanocomposites. Polym. Test. 2008, 27, 841–850. [Google Scholar] [CrossRef]
- Liu, M.; Guo, B.; Du, M.; Lei, Y.; Jia, D. Natural inorganic nanotubes reinforced epoxy resin nanocomposites. J. Polym. Res. 2008, 15, 205–212. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-targeted adsorption onto halloysite nanotubes in aqueous media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent advances on surface modification of halloysite nanotubes for multifunctional applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Berahman, R.; Raiati, M.; Mehrabi Mazidi, M.; Paran, S.M.R. Preparation and characterization of vulcanized silicone rubber/halloysite nanotube nanocomposites: Effect of matrix hardness and HNT content. Mater. Des. 2016, 104, 333–345. [Google Scholar] [CrossRef]
- Schmitt, H.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation and properties of novel melt-blended halloysite nanotubes/wheat starch nanocomposites. Carbohydr. Polym. 2012, 89, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chang, P.R.; Wang, S.; Yu, J.; Ma, X. Preparation and properties of halloysite nanotubes/plasticized Dioscorea opposita Thunb. starch composites. Carbohydr. Polym. 2011, 83, 186–191. [Google Scholar] [CrossRef]
- Avérous, L.; Fringant, C.; Moro, L. Plasticized starch–cellulose interactions in polysaccharide composites. Polymer 2001, 42, 6565–6572. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Ma, X. Study on the properties of ethylenebisformamide and sorbitol plasticized corn starch (ESPTPS). Carbohydr. Polym. 2006, 66, 110–116. [Google Scholar] [CrossRef]
- Tang, S.; Zou, P.; Xiong, H.; Tang, H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohydr. Polym. 2008, 72, 521–526. [Google Scholar] [CrossRef]
- Levis, S.R.; Deasy, P.B. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002, 243, 125–134. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on supramolecular structural changes of ultrasonic treated potato starch granules. Food Hydrocoll. 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Nuessli, J.; Putaux, J.L.; Bail, P.L.; Buléon, A. Crystal structure of amylose complexes with small ligands. Int. J. Biol. Macromol. 2003, 33, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Changes in the mechanical properties of thermoplastic potato starch in relation with changes in B-type crystallinity. Carbohydr. Polym. 1996, 29, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- He, Y.; Kong, W.; Wang, W.; Liu, T.; Liu, Y.; Gong, Q.; Gao, J. Modified natural halloysite/potato starch composite films. Carbohydr. Polym. 2012, 87, 2706–2711. [Google Scholar] [CrossRef]
- Anglès, M.N.; Dufresne, A. Plasticized starch/tunicin whiskers nanocomposites. 1. Structural analysis. Macromolecules 2000, 33, 8344–8353. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. Starch nano-biocomposites based on needle-like sepiolite clays. Carbohydr. Polym. 2010, 80, 145–153. [Google Scholar] [CrossRef]
- Liu, M.; Guo, B.; Du, M.; Chen, F.; Jia, D. Halloysite nanotubes as a novel β-nucleating agent for isotactic polypropylene. Polymer 2009, 50, 3022–3030. [Google Scholar] [CrossRef]
- Guo, B.; Zou, Q.; Lei, Y.; Du, M.; Liu, M.; Jia, D. Crystallization behavior of polyamide 6/halloysite nanotubes nanocomposites. Thermochim. Acta 2009, 484, 48–56. [Google Scholar] [CrossRef]
- Tang, X.-G.; Hou, M.; Zou, J.; Truss, R. Poly(vinylidene fluoride)/halloysite nanotubes nanocomposites: The structures, properties, and tensile fracture behaviors. J. Appl. Polym. Sci. 2013, 128, 869–878. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Nikkhah, S.J.; Ramazani, S.A.A.; Baniasadi, H.; Tavakolzadeh, F. Investigation of properties of polyethylene/clay nanocomposites prepared by new in situ Ziegler–Natta catalyst. Mater. Des. 2009, 30, 2309–2315. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Olivato, J.B.; Marini, J.; Pollet, E.; Yamashita, F.; Grossmann, M.V.E.; Avérous, L. Elaboration, morphology and properties of starch/polyester nano-biocomposites based on sepiolite clay. Carbohydr. Polym. 2015, 118, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, Y.; Li, X.; Chen, W. Synthesis of vegetable oil-based waterborne polyurethane/silver-halloysite antibacterial nanocomposites. Compos. Sci. Technol. 2016, 126, 86–93. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Dole, P.; Avérous, L. Starch-based nano-biocomposites: Plasticizer impact on the montmorillonite exfoliation process. Carbohydr. Polym. 2010, 79, 941–947. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Averous, L. Innovative plasticized alginate obtained by thermo-mechanical mixing: Effect of different biobased polyols systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.H. Plasticization of pea starch films with monosaccharides and polyols. J. Food Sci. 2006, 71, E253–E261. [Google Scholar] [CrossRef]
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of thermoplastic blends: Starch–polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]




| Sample Code | T10% (°C) | T50% (°C) | T90% (°C) | Tmax (°C) | Char Residue (%) |
|---|---|---|---|---|---|
| G0 | 131 | 291 | 337 | 287 | 0 |
| G3 | 161 | 293 | 373 | 289 | 2.1 |
| G5 | 195 | 295 | 463 | 291 | 4.9 |
| G7 | 194 | 298 | 511 | 306 | 7.0 |
| P0 | 189 | 297 | 361 | 288 | 0 |
| P3 | 230 | 301 | 401 | 288 | 3.1 |
| P5 | 226 | 300 | 429 | 290 | 4.6 |
| P7 | 232 | 300 | 496 | 304 | 7.1 |
| S0 | 223 | 297 | 349 | 297 | 0 |
| S3 | 239 | 300 | 401 | 298 | 2.2 |
| S5 | 248 | 300 | 448 | 299 | 4.7 |
| S7 | 245 | 300 | 467 | 306 | 6.3 |
| Glycerol | 127 | 154 | 162 | - | |
| Sorbitol | 218 | 251 | 268 | - | |
| Halloysite | 405 | >600 | >600 | - |
| Sample Code | Stress at Break (MPa) | Strain at Break (%) | Young’s Modulus (MPa) | Moisture Content (%) | Density (g/cm3) |
|---|---|---|---|---|---|
| G0 | 2.28 ± 0.13 | 26.1 ± 2.3 | 22.0 ± 2.2 | 16.7 ± 0.2 | 1.45 ± 0.02 |
| G3 | 2.64 ± 0.11 | 27.3 ± 2.6 | 28.3 ± 1.9 | 15.0 ± 0.2 | 1.47 ± 0.01 |
| G5 | 2.94 ± 0.05 | 29.3 ± 2.3 | 34.6 ± 2.2 | 14.2 ± 0.2 | 1.48 ± 0.01 |
| G7 | 3.36 ± 0.24 | 34.5 ± 3.6 | 37.0 ± 2.7 | 13.8 ± 0.2 | 1.50 ± 0.01 |
| P0 | 7.13 ± 0.06 | 42.4 ± 1.7 | 119.2 ± 4.9 | 11.1 ± 0.1 | 1.48 ± 0.01 |
| P3 | 7.36 ± 0.22 | 39.6 ± 1.9 | 144.7 ± 12.0 | 10.6 ± 0.1 | 1.49 ± 0.01 |
| P5 | 7.77 ± 0.12 | 37.0 ± 0.4 | 194.1 ± 15.3 | 10.4 ± 0.1 | 1.50 ± 0.01 |
| P7 | 7.88 ± 0.22 | 33.2 ± 3.0 | 174.5 ± 8.1 | 10.0 ± 0.1 | 1.52 ± 0.01 |
| S0 | 9.70 ± 0.79 | 43.3 ± 3.0 | 256.0 ± 19.0 | 9.6 ± 0.1 | 1.49 ± 0.03 |
| S3 | 10.24 ± 0.48 | 39.1 ± 2.7 | 276.5 ± 28.8 | 8.4 ± 0.1 | 1.50 ± 0.02 |
| S5 | 10.52 ± 0.51 | 36.5 ± 5.4 | 281.8 ± 19.0 | 8.7 ± 0.1 | 1.52 ± 0.01 |
| S7 | 10.78 ± 0.37 | 35.0 ± 5.6 | 292.1 ± 34.9 | 7.5 ± 0.1 | 1.54 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Dang, K.M.; Pollet, E.; Avérous, L. Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content. Polymers 2018, 10, 808. https://doi.org/10.3390/polym10080808
Ren J, Dang KM, Pollet E, Avérous L. Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content. Polymers. 2018; 10(8):808. https://doi.org/10.3390/polym10080808
Chicago/Turabian StyleRen, Jiawei, Khanh Minh Dang, Eric Pollet, and Luc Avérous. 2018. "Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content" Polymers 10, no. 8: 808. https://doi.org/10.3390/polym10080808