Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.2.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.3. Elemental Analysis
2.3. Synthesis of Chitosan Derivatives
2.3.1. Synthesis of N,N,N-Trimethyl Chitosan Iodide (TMCSI)
2.3.2. Synthesis of Quaternary Ammonium Salt of Chitosan Bearing Halogenated Acetate
2.4. Antifungal Assays
2.5. Antibacterial Assays
2.6. Statistical Analysis
3. Results and Discussion
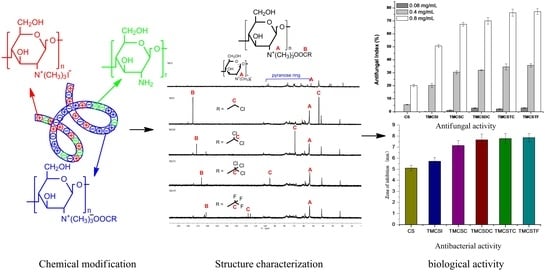
3.1. Structure of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate
3.2. Antifungal Activity
3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Tan, W.; Zhang, Z.; Song, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antifungal activity of chitosan derivatives containing urea groups. Int. J. Biol. Macromol. 2018, 109, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Asghar, M.A.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B 1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef]
- Yang, G.; Jin, Q.; Xu, C.; Fan, S.; Wang, C.; Xie, P. Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Lukowska-Chojnacka, E.; Mierzejewska, J.; Milner-Krawczyk, M.; Bondaryk, M.; Staniszewska, M. Synthesis of novel tetrazole derivatives and evaluation of their antifungal activity. Bioorg. Med. Chem. 2016, 24, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Morkaew, T.; Pinyakong, O.; Tachaboonyakiat, W. Structural effect of quaternary ammonium chitin derivatives on their bactericidal activity and specificity. Int. J. Biol. Macromol. 2017, 101, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zahir-Jouzdani, F.; Mahbod, M.; Soleimani, M.; Vakhshiteh, F.; Arefian, E.; Shahosseini, S.; Dinarvand, R.; Atyabi, F. Chitosan and thiolated chitosan: Novel therapeutic approach for preventing corneal haze after chemical injuries. Carbohydr. Polym. 2018, 179, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Phunpee, S.; Saesoo, S.; Sramala, I.; Jarussophon, S.; Sajomsang, W.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U.R. A comparison of eugenol and menthol on encapsulation characteristics with water-soluble quaternized beta-cyclodextrin grafted chitosan. Int. J. Biol. Macromol. 2016, 84, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Saesoo, S.; Ovatlarnporn, C. Antifungal property of quaternized chitosan and its derivatives. Int. J. Biol. Macromol. 2012, 50, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Chongprakobkit, S.; Maniratanachote, R.; Tachaboonyakiat, W. Oil-in-water emulsions stabilized by sodium phosphorylated chitosan. Carbohydr. Polym. 2013, 96, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromol. 2016, 92, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Chen, X.; Ji, X.; Li, P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Dong, F.; Xue, Q.; Wang, G.; Qin, S.; Guo, Z. The influence of the cation of quaternized chitosans on antioxidant activity. Carbohydr. Polym. 2009, 78, 439–443. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. The influence of the cationic of quaternized chitosan on antifungal activity. Int. J. Food Microbiol. 2007, 118, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, J.; Li, R.; Dong, F.; Guo, Z. Antifungal properties of chitosan salts in laboratory media. J. Appl. Polym. Sci. 2012, 124, 2501–2507. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.S.; Peng, X.M.; Kishore, B.; Zhou, C.H. 1,2,3-Triazole-derived naphthalimides as a novel type of potential antimicrobial agents: Synthesis, antimicrobial activity, interaction with calf thymus DNA and human serum albumin. Bioorg. Med. Chem. Lett. 2014, 24, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Aktan, E.; Gündüzalp, A.B.; Özmen, Ü.Ö. Structural, physicochemical characterization, theoretical studies of carboxamides and their Cu(II), Zn(II) complexes having antibacterial activities against E. coli. J. Mol. Struct. 2017, 1128, 775–784. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal evaluation of novel 1,2,3-triazolium-functionalized starch derivative. Int. J. Biol. Macromol. 2017, 101, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Gao, Z.; Qiu, S.; Dong, F.; Guo, Z. Design, synthesis of novel starch derivative bearing 1,2,3-triazolium and pyridinium and evaluation of its antifungal activity. Carbohydr. Polym. 2017, 157, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Pandey, J.; Kumar, P. Synthesis and characterization of modified chitosan via microwave route for novel antibacterial application. Int. J. Biol. Macromol. 2018, 107, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Dhanavel, S.; Manivannan, N.; Mathivanan, N.; Gupta, V.K.; Narayanan, V.; Stephen, A. Preparation and characterization of cross-linked chitosan/palladium nanocomposites for catalytic and antibacterial activity. J. Mol. Liq. 2018, 257, 32–41. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Tang, F.; Lv, L.; Lu, F.; Rong, B.; Li, Z.; Lu, B.; Yu, K.; Liu, J.; Dai, F.; Wu, D.; et al. Preparation and characterization of N-chitosan as a wound healing accelerator. Int. J. Biol. Macromol. 2016, 93, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Almada, M.; Leal-Martinez, B.H.; Hassan, N.; Kogan, M.J.; Burboa, M.G.; Topete, A.; Valdez, M.A.; Juarez, J. Photothermal conversion efficiency and cytotoxic effect of gold nanorods stabilized with chitosan, alginate and poly(vinyl alcohol). Mater. Sci. Eng. C 2017, 77, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Saeeduddin, M.; Zeng, X. Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr. Polym. 2017, 170, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Wang, H.; Liu, Y.; Zhang, J.; Dong, F.; Guo, Z. Synthesis, characterization, and antibacterial property of novel starch derivatives with 1,2,3-triazole. Carbohydr. Polym. 2016, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, Z.; Jiang, P. Synthesis, characterization, and antifungal activity of novel quaternary chitosan derivatives. Carbohydr. Res. 2010, 345, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Li, W.; Dong, F.; Guo, Z. Synthesis and antioxidant property of novel 1,2,3-triazole-linked starch derivatives via “click chemistry”. Int. J. Biol. Macromol. 2016, 82, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Dananjaya, S.H.S.; Erandani, W.; Kim, C.H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel thiosemicarbazone chitosan derivatives: Preparation, characterization, and antifungal activity. Carbohydr. Polym. 2012, 87, 2664–2670. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B.; Kyzioł, A.; Wydro, P. Chitosan as a subphase disturbant of membrane lipid monolayers. The effect of temperature at varying pH: II. DPPC and cholesterol. Coll. Surf. A 2013, 434, 359–364. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Dong, F.; Luan, F.; Guo, Z. The influence of starch derivatives with benzene or halogenated benzene on antibacterial activity. Starch Stärke 2017, 69, 1600350. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Tantayanon, S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: Preparation and characterization. Int. J. Biol. Macromol. 2009, 44, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Du, Y.; Fan, L.; Chen, X.; Yang, J. Preparation, characterization and antimicrobial activity of quaternized carboxymethyl chitosan and application as pulp-cap. Polymer 2006, 47, 1796–1804. [Google Scholar] [CrossRef]







| Compounds | Yields (%) | Elemental Analyses (%) | Degrees of Substitution | Deacetylation | |||
|---|---|---|---|---|---|---|---|
| C | N | H | C/N | ||||
| CS | 40.851 | 7.474 | 6.879 | 5.47 | - | 0.81 | |
| TMCSI | 87.6 | 29.211 | 4.076 | 5.485 | 7.17 | 0.66 | - |
| TMCSC | 73.5 | 39.28 | 4.941 | 6.461 | 7.95 | 0.46 | - |
| TMCSDC | 69.8 | 38.439 | 4.689 | 7.217 | 8.2 | 0.6 | - |
| TMCSTC | 75.3 | 43.054 | 5.361 | 7.837 | 8.03 | 0.5 | - |
| TMCSTF | 74.6 | 42.88 | 5.556 | 6.998 | 7.72 | 0.32 | - |
| Microorganism | Zone of Inhibition in mm (Mean ± SD) n = 3 | |||||
|---|---|---|---|---|---|---|
| CS | TMCSI | TMCSC | TMCSDC | TMCSTC | TMCSTF | |
| E. coli | 5.11 ± 0.25 | 5.72 ± 0.35 | 7.14 ± 0.46 | 7.66 ± 0.52 | 7.77 ± 0.47 | 7.86 ± 0.39 |
| S. aureus | 5.19 ± 0.21 | 6.42 ± 0.53 | 6.62 ± 0.48 | 7.36 ± 0.60 | 8.23 ± 0.49 | 8.77 ± 0.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Tan, W.; Luan, F.; Yin, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities. Polymers 2018, 10, 530. https://doi.org/10.3390/polym10050530
Zhang J, Tan W, Luan F, Yin X, Dong F, Li Q, Guo Z. Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities. Polymers. 2018; 10(5):530. https://doi.org/10.3390/polym10050530
Chicago/Turabian StyleZhang, Jingjing, Wenqiang Tan, Fang Luan, Xiuli Yin, Fang Dong, Qing Li, and Zhanyong Guo. 2018. "Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities" Polymers 10, no. 5: 530. https://doi.org/10.3390/polym10050530