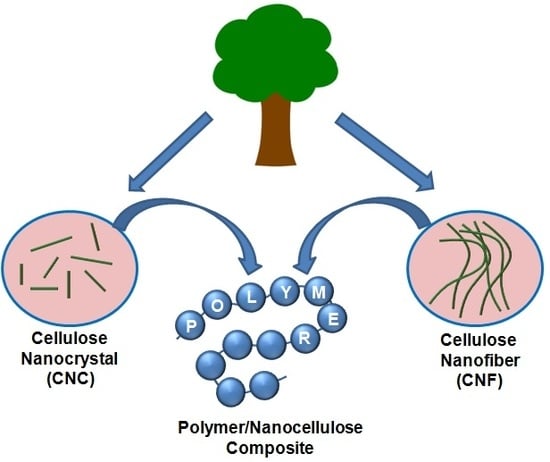
Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them
Abstract
:1. Introduction
2. Nanocellulose: Availability and Properties
2.1. Cellulose Nanocrystal (CNC)
2.2. Cellulose Nanofiber (CNF)
3. Polymer Nanocomposites Reinforced by CNC
3.1. Polymer/CNC Nanocomposites with Covalent Interaction
3.1.1. Urethanization
3.1.2. Etherification
3.1.3. Peptide Coupling
3.1.4. Silane Coupling
3.1.5. Click Reaction
3.1.6. Surface-Initiated Radical Polymerization
3.1.7. Ring-Opening Polymerization
3.1.8. Esterification
3.2. Polymer/CNC Nanocomposites with Non-Covalent Interactions
3.2.1. Hydrogen Bonding
3.2.2. Electrostatic Interaction
3.2.3. Physisorption
3.2.4. Compatibilization through Surface Modification
4. Polymer Nanocomposites Reinforced by CNF
4.1. Polymer/CNF Nanocomposite with Covalent Interactions
4.1.1. Urethanization
4.1.2. Silane Coupling
4.1.3. Etherification
4.1.4. Peptidic Coupling
4.1.5. Esterification
4.1.6. Surface-Initiated Polymerization
4.2. Polymer/CNF Nanocomposite with Non-Covalent Interactions
4.2.1. Electrostatic Interaction
4.2.2. Hydrogen-Bonding Interaction
4.2.3. Compatibilization through Surface Modification
4.2.4. Pickering Emulsion
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated cellulose surface modification: A review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Nishio, Y. Material Functionalization of Cellulose and Related Polysaccharides via Diverse Microcompositions. In Polysaccharides II; Klemm, D., Ed.; Springer: Berlin, Germany, 2006; Volume 205, pp. 97–151. [Google Scholar]
- Nishio, Y.; Teramoto, Y.; Kusumi, R.; Sugimura, K.; Aranishi, Y. Blends and Graft Copolymers of Cellulosics: Toward the Design and Development of Advanced Films and Fibers; Springer: Berlin, Germany, 2017. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Ray, D.; Sain, S. In situ processing of cellulose nanocomposites. Composites Part A 2016, 83, 19–37. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Composites Part A 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, E.; Carlmark, A. Controlled grafting of cellulose fibres—An outlook beyond paper and cardboard. Polym. Chem. 2012, 3, 1702–1713. [Google Scholar] [CrossRef]
- Brown, R.M. The biosynthesis of cellulose. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, A33, 1345–1373. [Google Scholar] [CrossRef]
- Jarvis, M.C. Structure of native cellulose microfibrils, the starting point for nanocellulose manufacture. Philos. Trans. R. Soc. A 2018, 376, 20170045. [Google Scholar] [CrossRef] [PubMed]
- Mozdyniewicz, D.J.; Nieminen, K.; Kraft, G.; Sixta, H. Degradation of viscose fibers during acidic treatment. Cellulose 2016, 23, 213–229. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Yano, H. Production of cellulose nanofibres and their applications. Nippon Gomu Kyokaishi 2012, 12, 376–381. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Rånby, B.G. Aqueous Colloidal Solutions of Cellulose Micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Mukherjee, S.M.; Woods, H.J. X-ray and electron microscope studies of the degradation of cellulose by sulphuric acid. Biochim. Biophys. Acta 1953, 10, 499–511. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E.; Schwegler-Berry, D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009, 11, 1808–1814. [Google Scholar] [CrossRef]
- Hayashi, N.; Kondo, T.; Ishihara, M. Enzymatically produced nano-ordered short elements containing cellulose Iβ crystalline domains. Carbohydr. Polym. 2005, 61, 191–197. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Angles, M.N.; Dufresne, A. Plasticized starch/tunicin whiskers nanocomposite materials. 2. mechanical behavior. Macromolecules 2001, 34, 2921–2931. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Espinosa, S.C.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Garcia de Rodriguez, N.; Thielemans, W.; Dufresne, A. Sisal cellulose whiskers reinforced polyvinyl acetate nanocomposites. Cellulose 2006, 13, 261–270. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Paillet, M.; Dufresne, A. Tangling effect in fibrillated cellulose reinforced nanocomposites. Macromolecules 2004, 37, 4313–4316. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S. Effect of trace electrolyte on liquid crystal type of cellulose microcrystals. Langmuir 2001, 17, 4493–4496. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Cellulose isolation and core-shell nanostructures of cellulose nanocrystals from chardonnay grape skins. Carbohydr. Polym. 2012, 87, 2546–2553. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Flauzino Neto, W.P.; Mariano, M.; da Silva, I.S.V.; Silvério, H.A.; Putaux, J.-L.; Otaguro, H.; Pasquini, D.; Dufresne, A. Mechanical properties of natural rubber nanocomposites reinforced with high aspect ratio cellulose nanocrystals isolated from soy hulls. Carbohydr. Polym. 2016, 153, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of cellulose nanocrystals for advanced applications. J. Colloid Interface Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. In Proceedings of the Ninth Cellulose Conference, Syracuse, NY, USA, 24–27 May 1982; Volume 37, pp. 797–813. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties uses, and commercial potential. In Proceedings of the Ninth Cellulose Conference, Syracuse, NY, USA, 24–27 May 1982; Volume 37, pp. 815–827. [Google Scholar]
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose fibrils for polymer reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr. Polym. 2014, 112, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Okamura, K. New films produced from microfibrillated natural fibres. Polym. Int. 1998, 47, 291–294. [Google Scholar] [CrossRef]
- Iwamoto, S.; Abe, K.; Yano, H. The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules 2008, 9, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Sain, M.; Kortschot, M. Reinforcing potential of wood pulp-derived microfibres in a PVA matrix. Holzforschung 2006, 60, 53–58. [Google Scholar] [CrossRef]
- Uetani, K.; Yano, H. Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 2010, 12, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Homma, I.; Matsubara, Y. Complete nanofibrillation of cellulose prepared by phosphorylation. Cellulose 2017, 24, 1295–1305. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. J. Appl. Polym. Sci. 2011, 119, 2652–2660. [Google Scholar] [CrossRef]
- Iwamoto, S.; Endo, T. 3 Nm Thick Lignocellulose Nano Fibers Obtained From Esterified Wood With Maleic Anhydride. ACS Macro Lett. 2015, 4, 80–83. [Google Scholar] [CrossRef]
- Ho, T.T.T.; Zimmermann, T.; Hauert, R.; Caseri, W. Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes. Cellulose 2011, 18, 1391–1406. [Google Scholar] [CrossRef]
- Eyholzer, C.; Bordeanu, N.; Lopez-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 2010, 17, 19–30. [Google Scholar] [CrossRef]
- Missoum, K.; Bras, J.; Belgacem, M.N. Water redispersible dried nanofibrillated cellulose by adding sodium chloride. Biomacromolecules 2012, 13, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Hiasa, S.; Kumagai, A.; Endo, T.; Edashige, Y. Prevention of aggregation of pectin-containing cellulose nanofibers prepared from mandarin peel. J. Fiber Sci. Technol. 2016, 72, 17–26. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Suresh kumar, P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef]
- Zhao, Y.; Moser, C.; Lindstrom, M.E.; Henriksson, G.; Li, J. Cellulose nanofibers from softwood, hardwood, and tunicate: Preparation–structure–film performance interrelation. ACS Appl. Mater. Interfaces 2017, 9, 13508–13519. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Cherian, B.M.; Bismarck, A.; Blaker, J.J.; Pothan, L.A.; Leao, A.L.; de Souza, S.F.; Kottaisamy, M. Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Comparison of the characteristics of cellulose microfibril aggregates isolated from fiber and parenchyma cells of Moso bamboo (Phyllostachys pubescens). Cellulose 2010, 17, 271–277. [Google Scholar] [CrossRef]
- De Morais Teixeira, E.; Corrêa, A.C.; Manzoli, A.; de Lima Leite, F.; de Ribeiro Oliveira, C.; Mattoso, L.H.C. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Ek, R.; Gustafsson, C.; Nutt, A.; Iversen, T.; Nyström, C. Cellulose powder from Cladophora sp. Algae. J. Mol. Recognit. 1998, 11, 263–265. [Google Scholar] [CrossRef]
- Jonoobi, M.; Mathew, A.P.; Oksman, K. Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind. Crops Prod. 2012, 40, 232–238. [Google Scholar] [CrossRef]
- Berglund, L.; Noël, M.; Aitomäki, Y.; Öman, T.; Oksman, K. Production potential of cellulose nanofibers from industrial residues: Efficiency and nanofiber characteristics. Ind. Crops Prod. 2016, 92, 84–92. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Comparing the mechanical properties of high performances polymer nanocomposites from biological sources. J. Nanosci. Nanotechnol. 2006, 6, 322–330. [Google Scholar] [CrossRef]
- De Sousa Lima, M.M.; Borsali, R. Rodlike cellulose microcrystals: Structure, properties, and applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- Dufresne, A. Interfacial phenomena in nanocomposites based on polysaccharide nanocrystals. Compos. Interfaces 2003, 10, 369–387. [Google Scholar] [CrossRef]
- Habibi, Y.; Dufresne, A. Highly filled bionanocomposites from functionalized polysaccharides nanocrystals. Biomacromolecules 2008, 9, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Yang, G.; Zheng, X.; Zhou, S. Multi-stimulus-responsive shape-memory polymer nanocomposite network cross-linked by cellulose nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 4118–4126. [Google Scholar] [CrossRef] [PubMed]
- Girouard, N.M.; Xu, S.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Site-selective modification of cellulose nanocrystals with isophorone diisocyanate and formation of polyurethane-CNC composites. ACS Appl. Mater. Interfaces 2016, 8, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Lee, S.-Y. Physiochemical, optical and mechanical properties of poly(lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals. RSC Adv. 2016, 6, 9438–9445. [Google Scholar] [CrossRef]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Li, M.; Lee, S.-Y. Mechanical and thermal properties of toluene diisocyanate-modified cellulose nanocrystal nanocomposites using semi-crystalline poly(lactic acid) as a base matrix. RSC Adv. 2016, 6, 73879–73886. [Google Scholar] [CrossRef]
- Sethi, J.; Illikainen, M.; Sain, M.; Oksman, K. Polylactic acid/polyurethane blend reinforced with cellulose nanocrystals with semi-interpenetrating polymer network (S-IPN) structure. Eur. Polym. J. 2017, 86, 188–199. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Kloser, E.; Gray, D.G. Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Azzam, F.; Heux, L.; Putaux, J.-L.; Jean, B. Preparation by grafting onto, characterization and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- C˘er˘ovský, V.; Jakubke, H.D. Peptide synthesis catalyzed by crosslinked α-chymotrypsin in water/dimethylformamide solvent system. Biocatalysis 1994, 11, 233–240. [Google Scholar] [CrossRef]
- Cudjoe, E.; Khani, S.; Way, A.E.; Hore, M.J.A.; Maia, J.; Rowan, S.J. Biomimetic Reversible Heat-Stiffening Polymer Nanocomposites. ACS Cent. Sci. 2017, 3, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.B.; Salon, M.C.B.; Magnin, A.; Belgacem, M.N.; Boufi, S. Cellulose-based nanocomposites prepared via mini-emulsion polymerization: Understanding the chemistry of the nanocellulose/matrix interface. Colloids Surf. A 2014, 448, 1–8. [Google Scholar] [CrossRef]
- Ladhar, A.; Ben Mabrouk, A.; Arous, M.; Boufi, S.; Kallel, A. Dielectric properties of nanocomposites based on cellulose nanocrystals (CNCs) and poly(styrene-co-2-ethyl hexylacrylate) copolymer. Polymer 2017, 125, 76–89. [Google Scholar] [CrossRef]
- Rahmat, M.; Karrabi, M.; Ghasemi, I.; Zandi, M.; Azizi, H. Silane crosslinking of electrospun poly(lactic acid)/nanocrystalline cellulose bionanocomposite. Mater. Sci. Eng., C 2016, 68, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C.; Xie, X.-M. Studies on the properties and formation mechanism of flexible nanocomposite hydrogels from cellulose nanocrystals and poly(acrylic acid). J. Mater. Chem. 2012, 22, 22467–22480. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C. Synthesis and characterization of mechanically flexible and tough cellulose nanocrystals-polyacrylamide nanocomposite hydrogels. Cellulose 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Chang, H.; Xu, F.; Yang, J. A self-healing cellulose nanocrystal-poly(ethylene glycol) nanocomposite hydrogel via diels-alder click reaction. ACS Sustain. Chem. Eng. 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly[2-(dimethylamino)ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Spinella, S.; Samuel, C.; Raquez, J.-M.; McCallum, S.A.; Gross, R.; Dubois, P. Green and efficient synthesis of dispersible cellulose nanocrystals in biobased polyesters for engineering applications. ACS Sustain. Chem. Eng. 2016, 4, 2517–2527. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Graham, L.; Moorlag, C.; Dooley, B.M.; Cranston, E.D. Poly(methyl methacrylate)-grafted cellulose nanocrystals: One-step synthesis, nanocomposite preparation, and characterization. Can. J. Chem. Eng. 2016, 94, 811–822. [Google Scholar] [CrossRef]
- Tang, J.; Berry, R.M.; Tam, K.C. Stimuli-Responsive Cellulose Nanocrystals for Surfactant-Free Oil Harvesting. Biomacromolecules 2016, 17, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Pracella, M.; Haque, M.M.-U.; Puglia, D. Morphology and properties tuning of PLA/cellulose nanocrystals bio-nanocomposites by means of reactive functionalization and blending with PVAc. Polymer 2014, 55, 3720–3728. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through surface-initiated atom transfer radical polymerization (SI-ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, C.; Wang, J.; Chu, F. In situ development of self-reinforced cellulose nanocrystals based thermoplastic elastomers by atom transfer radical polymerization. Carbohydr. Polym. 2016, 141, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Boujemaoui, A.; Sanchez, C.C.; Engström, J.; Bruce, C.; Fogelström, L.; Carlmark, A.; Malmström, E. Polycaprolactone nanocomposites reinforced with cellulose nanocrystals surface-modified via covalent grafting or physisorption: A comparative study. ACS Appl. Mater. Interfaces 2017, 9, 35305–35318. [Google Scholar] [CrossRef] [PubMed]
- Hatton, F.L.; Kedzior, S.A.; Cranston, E.D.; Carlmark, A. Grafting-from cellulose nanocrystals via photoinduced Cu-mediated reversible-deactivation radical polymerization. Carbohydr. Polym. 2017, 157, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Habibi, Y.; Rojas, O.J.; Venditti, R.A.; Johansson, L.-S.; Efimenko, K.; Österberg, M.; Laine, J. Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 2010, 11, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Osterberg, M.; Venditti, R.A.; Laine, J.; Rojas, O.J. Surface interaction forces of cellulose nanocrystals grafted with thermoresponsive polymer brushes. Biomacromolecules 2011, 12, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Venditti, R.A.; Rojas, O.J. Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J. Colloid Interface Sci. 2012, 369, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Nuyken, O.; Pask, S.D. Ring-opening polymerization-An introductory review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. In-situ polymerized cellulose nanocrystals (CNC)-poly(l-lactide) (PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr. Polym. 2016, 153, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; León, L.M.; Armentano, I.; Torre, L.; Kenny, J.M. PLLA-grafted cellulose nanocrystals: Role of the CNC content and grafting on the PLA bionanocomposite film properties. Carbohydr. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef] [PubMed]
- De Paula, E.L.; Roig, F.; Mas, A.; Habas, J.-P.; Mano, V.; Pereira, F.V.; Robin, J.-J. Effect of surface-grafted cellulose nanocrystals on the thermal and mechanical properties of PLLA based nanocomposites. Eur. Polym. J. 2016, 84, 173–187. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Zhou, L.; Duan, Y.; Zhang, J. Synthesis and characterization of cellulose nanocrystal-graft-poly(D-lactide) and its nanocomposite with poly(l-lactide). Polymer 2016, 103, 365–375. [Google Scholar] [CrossRef]
- Chen, J.; Wu, D.; Tam, K.C.; Pan, K.; Zheng, Z. Effect of surface modification of cellulose nanocrystal on nonisothermal crystallization of poly(β-hydroxybutyrate) composites. Carbohydr. Polym. 2017, 157, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly biodegradable and tough polylactic acid-cellulose nanocrystal composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Mannan, T.M.; Soares, J.B.P.; Berry, R.M.; Hamad, W.Y. In-situ production of polyethylene/cellulose nanocrystal composites. Can. J. Chem. Eng. 2016, 94, 2107–2113. [Google Scholar] [CrossRef]
- Goetz, L.; Mathew, A.; Oksman, K.; Gatenholm, P.; Ragauskas, A.J. A novel nanocomposite film prepared from crosslinked cellulosic whiskers. Carbohydr. Polym. 2009, 75, 85–89. [Google Scholar] [CrossRef]
- Kashani Rahimi, S.; Aeinehvand, R.; Kim, K.; Otaigbe, J.U. Structure and biocompatibility of bioabsorbable nanocomposites of aliphatic-aromatic copolyester and cellulose nanocrystals. Biomacromolecules 2017, 18, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Girones, J.; Vo, L.T.T.; Haudin, L.-M.; Freire, L.; Navard, P. Crystallization of polypropylene in the presence of biomass-based fillers of different compositions. Polymer 2017, 127, 220–231. [Google Scholar] [CrossRef]
- Zhu, B.; Merindol, R.; Benitez, A.; Wang, B.; Walther, A. Supramolecular engineering of hierarchically self-assembled, bioinspired, cholesteric nanocomposites formed by cellulose nanocrystals and polymers. ACS Appl. Mater. Interfaces 2016, 8, 11031–11040. [Google Scholar] [CrossRef] [PubMed]
- Kiziltas, E.E.; Kiziltas, A.; Bollin, S.C.; Gardner, D.J. Preparation and characterization of transparent PMMA-cellulose-based nanocomposites. Carbohydr. Polym. 2015, 127, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Bhasney, S.M.; Kumar, A.; Katiyar, V. Acid functionalized cellulose nanocrystals and its effect on mechanical, thermal, crystallization and surfaces properties of poly(lactic acid) bionanocomposites films: A comprehensive study. Polymer 2016, 101, 75–92. [Google Scholar] [CrossRef]
- Meree, C.E.; Schueneman, G.T.; Meredith, J.C.; Shofner, M.L. Rheological behavior of highly loaded cellulose nanocrystal/poly(vinyl alcohol) composite suspensions. Cellulose 2016, 23, 3001–3012. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 42004. [Google Scholar] [CrossRef]
- El Miri, N.; El Achaby, M.; Fihri, A.; Larzek, M.; Zahouily, M.; Abdelouahdi, K.; Barakat, A.; Solhy, A. Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites. Carbohydr. Polym. 2016, 137, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.P.; Shofner, M.L. Processing strategies for cellulose nanocrystal/polyethylene-co-vinyl alcohol composites. Polymer 2017, 126, 211–223. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Surov, O.V.; Voronova, M.I.; Afineevskii, A.V.; Zakharov, A.G. Polyethylene oxide films reinforced by cellulose nanocrystals: Microstructure-properties relationship. Carbohydr. Polym. 2018, 181, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Stimpson, T.C.; Niinivaara, E.; Villalobos, M.; Cranston, E.D. Comparing Soft Semicrystalline Polymer Nanocomposites Reinforced with Cellulose Nanocrystals and Fumed Silica. Ind. Eng. Chem. Res. 2018, 57, 220–230. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Mechanical and viscoelastic properties of cellulose nanocrystals reinforced poly(ethylene glycol) nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Li, Y.; Liu, Y.; Zhang, R.; Hsiao, B.S.; Zhu, M. Continuous fabrication of cellulose nanocrystal/poly(ethylene glycol) diacrylate hydrogel fiber from nanocomposite dispersion: Rheology, preparation and characterization. Polymer 2017, 123, 55–64. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, M.; Feng, J.; Zhang, S.; Wang, X. High oxygen barrier property of poly(propylene carbonate)/polyethylene glycol nanocomposites with low loading of cellulose nanocrytals. ACS Sustain. Chem. Eng. 2017, 5, 11246–11254. [Google Scholar] [CrossRef]
- De France, K.J.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced Mechanical Properties in Cellulose Nanocrystal-Poly(oligoethylene glycol methacrylate) Injectable Nanocomposite Hydrogels through Control of Physical and Chemical Cross-Linking. Biomacromolecules 2016, 17, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Meng, Q.; Bulone, V.; Zhou, Q. Flexible and responsive chiral nematic cellulose nanocrystal/poly(ethylene glycol) composite films with uniform and tunable structural color. Adv. Mater. 2017, 29, 1701323. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Jiang, C.; Liu, D.; Prempeh, N.; Smalyukh, I.I. Cellulose nanocrystal/poly(ethylene glycol) composite as an iridescent coating on polymer substrates: Structure-color and interface adhesion. ACS Appl. Mater. Interfaces 2016, 8, 32565–32573. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Kam, D.; Nevo, Y.; Slattegard, R.; Rivkin, A.; Lapidot, S.; Shoseyov, O. Highly modified cellulose nanocrystals and formation of epoxy-nanocrystalline cellulose (CNC) nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 28086–28095. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, D.; Lv, Q.; Yan, L. Crystallization temperature as the probe to detect polymer-filler compatibility in the poly(ε-caprolactone) composites with acetylated cellulose nanocrystal. J. Phys. Chem. C 2017, 121, 18615–18624. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystal reinforced oxidized natural rubber nanocomposites. Carbohydr. Polym. 2016, 137, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhen, X.; Wang, Z.; Zou, H.; Zhang, L.; Ning, N. Bioderived Rubber-Cellulose Nanocrystal Composites with Tunable Water-Responsive Adaptive Mechanical Behavior. ACS Appl. Mater. Interfaces 2017, 9, 6482–6487. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Echart, A.; Ugarte, L.; Arbelaiz, A.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Modulating the microstructure of waterborne polyurethanes for preparation of environmentally friendly nanocomposites by incorporating cellulose nanocrystals. Cellulose 2017, 24, 823–834. [Google Scholar] [CrossRef]
- Song, L.; Wang, Z.; Lamm, M.E.; Yuan, L.; Tang, C. Supramolecular polymer nanocomposites derived from plant oils and cellulose nanocrystals. Macromolecules 2017, 50, 7475–7483. [Google Scholar] [CrossRef]
- Seoane, I.T.; Fortunati, E.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Development and characterization of bionanocomposites based on poly(3-hydroxybutyrate) and cellulose nanocrystals for packaging applications. Polym. Int. 2016, 65, 1046–1053. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.-Y.; Song, M.-L.; Zhou, Y.; Yao, J.; Ni, Q.-Q. In vitro degradation and possible hydrolytic mechanism of PHBV nanocomposites by incorporating cellulose nanocrystal-ZnO nanohybrids. Carbohydr. Polym. 2017, 176, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ben Mabrouk, A.; Rei Vilar, M.; Magnin, A.; Belgacem, M.N.; Boufi, S. Synthesis and characterization of cellulose whiskers/polymer nanocomposite dispersion by mini-emulsion polymerization. J. Colloid Interface Sci. 2011, 363, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Heim, T.; Douillard, R. AC Electric Field-Assisted Assembly and Alignment of Cellulose Nanocrystals. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1430–1436. [Google Scholar] [CrossRef]
- Ten, E.; Jiang, L.; Wolcott, M.P. Preparation and properties of aligned poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites. Carbohydr. Polym. 2013, 92, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Vollick, B.; Kuo, P.-Y.; Alizadehgiashi, M.; Yan, N.; Kumacheva, E. From structure to properties of composite films derived from cellulose nanocrystals. ACS Omega 2017, 2, 5928–5934. [Google Scholar] [CrossRef]
- Sundman, O. Adsorption of four non-ionic cellulose derivatives on cellulose model surfaces. Cellulose 2014, 21, 115–124. [Google Scholar] [CrossRef]
- McKee, J.R.; Hietala, S.; Seitsonen, J.; Laine, J.; Kontturi, E.; Ikkala, O. Thermoresponsive nanocellulose hydrogels with tunable mechanical properties. ACS Macro Lett. 2014, 3, 266–270. [Google Scholar] [CrossRef]
- Hu, Z.; Patten, T.; Pelton, R.; Cranston, E.D. Synergistic stabilization of emulsions and emulsion gels with water-soluble polymers and cellulose nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 1023–1031. [Google Scholar] [CrossRef]
- Hu, Z.; Marway, H.S.; Kasem, H.; Pelton, R.; Cranston, E.D. Dried and Redispersible Cellulose Nanocrystal Pickering Emulsions. ACS Macro Lett. 2016, 5, 185–189. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Dube, M.A.; Cranston, E.D. Cellulose nanocrystals and methyl cellulose as costabilizers for nanocomposite latexes with double morphology. ACS Sustain. Chem. Eng. 2017, 5, 10509–10517. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, R.; Cranston, E.D.; Pelton, R.H. Stable aqueous foams from cellulose nanocrystals and methyl cellulose. Biomacromolecules 2016, 17, 4095–4099. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.-W. Isolation of cellulose nanocrystals from grain straws and their use for the preparation of carboxymethyl cellulose-based nanocomposite films. Carbohydr. Polym. 2016, 150, 187–200. [Google Scholar] [CrossRef] [PubMed]
- El Miri, N.; Abdelouahdi, K.; Barakat, A.; Zahouily, M.; Fihri, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films reinforced with cellulose nanocrystals: Rheology of film-forming solutions, transparency, water vapor barrier and tensile properties of films. Carbohydr. Polym. 2015, 129, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.S.F.; Battirola, L.C.; da Silva, L.C.E.; Gonçalves, M.C. Morphological investigation of cellulose acetate/cellulose nanocrystal composites obtained by melt extrusion. J. Appl. Polym. Sci. 2016, 133, 44201. [Google Scholar] [CrossRef]
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-enhanced cellulose nanocrystal Pickering emulsions. J. Colloid Interface Sci. 2015, 439, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.M.; Rodriguez, R.S.; Devilbiss, M.N.; Woodcock, J.W.; Davis, C.S.; Sinko, R.; Keten, S.; Gilman, J.W. Simultaneously tailoring surface energies and thermal stabilities of cellulose nanocrystals using ion exchange: Effects on polymer composite properties for transportation, infrastructure, and renewable energy applications. ACS Appl. Mater. Interfaces 2016, 8, 27270–27281. [Google Scholar] [CrossRef] [PubMed]
- Mariano, M.; Pilate, F.; de Oliveira, F.B.; Khelifa, F.; Dubois, P.; Raquez, J.-M.; Dufresne, A. Preparation of Cellulose Nanocrystal-Reinforced Poly(lactic acid) Nanocomposites through Noncovalent Modification with PLLA-Based Surfactants. ACS Omega 2017, 2, 2678–2688. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; El Kissi, N.; Dufresne, A. Ionic compatibilization of cellulose nanocrystals with quaternary ammonium salt and their melt extrusion with polypropylene. ACS Appl. Mater. Interfaces 2016, 8, 8755–8764. [Google Scholar] [CrossRef] [PubMed]
- Kaboorani, A.; Auclair, N.; Riedl, B.; Landry, V. Physical and morphological properties of UV-cured cellulose nanocrystal (CNC) based nanocomposite coatings for wood furniture. Prog. Org. Coat. 2016, 93, 17–22. [Google Scholar] [CrossRef]
- Inai, N.H.; Lewandowska, A.E.; Ghita, O.R.; Eichhorn, S.J. Interfaces in polyethylene oxide modified cellulose nanocrystal–polyethylene matrix composites. Compos. Sci. Technol. 2018, 154, 128–135. [Google Scholar] [CrossRef]
- Azouz, K.B.; Ramires, E.C.; Van den Fonteyne, W.; El Kissi, N.; Dufresne, A. Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macro Lett. 2012, 1, 236–240. [Google Scholar]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunati, E.; Gigli, M.; Luzi, F.; Dominici, F.; Lotti, N.; Gazzano, M.; Cano, A.; Chiralt, A.; Munari, A.; Kenny, J.M.; et al. Processing and characterization of nanocomposite based on poly(butylene/triethylene succinate) copolymers and cellulose nanocrystals. Carbohydr. Polym. 2017, 165, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Meesorn, W.; Shirole, A.; Vanhecke, D.; de Espinosa, L.M.; Weder, C. A simple and versatile strategy to improve the mechanical properties of polymer nanocomposites with cellulose nanocrystals. Macromolecules 2017, 50, 2364–2374. [Google Scholar] [CrossRef]
- Ludueña, L.N.; Fortunati, E.; Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Puglia, D.; Manfredi, L.B.; Pracella, M. Preparation and characterization of polybutylene-succinate/poly(ethylene-glycol)/cellulose nanocrystals ternary composites. J. Appl. Polym. Sci. 2016, 133, 43302. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Bras, J.; Fortunati, E.; Kenny, J.M.; Torre, L.; Lopez-Manchado, M.A. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites Part I. Processing and morphology. Carbohydr. Polym. 2013, 96, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Bitinis, N.; Fortunati, E.; Verdejo, R.; Bras, J.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites. Part II: Properties evaluation. Carbohydr. Polym. 2013, 96, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Morelli, C.L.; Belgacem, N.; Bretas, R.E.S.; Bras, J. Melt extruded nanocomposites of polybutylene adipate-co-terephthalate (PBAT) with phenylbutyl isocyanate modified cellulose nanocrystals. J. Appl. Polym. Sci. 2016, 133, 43678. [Google Scholar] [CrossRef]
- Biyani, M.V.; Weder, C.; Foster, E.J. Photoswitchable nanocomposites made from coumarin-functionalized cellulose nanocrystals. Polym. Chem. 2014, 5, 5501–5508. [Google Scholar] [CrossRef]
- Biyani, M.V.; Foster, E.J.; Weder, C. Light-healable supramolecular nanocomposites based on modified cellulose nanocrystals. ACS Macro Lett. 2013, 2, 236–240. [Google Scholar] [CrossRef]
- Natterodt, J.C.; Sapkota, J.; Foster, E.J.; Weder, C. Polymer nanocomposites with cellulose nanocrystals featuring adaptive surface groups. Biomacromolecules 2017, 18, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Natterodt, J.; Sapkota, J.; Goseki, R.; Weder, C.; Takahara, A.; Otsuka, H. Dynamic covalent diarylbibenzofuranone-modified nanocellulose: Mechanochromic behaviour and application in self-healing polymer composites. Polym. Chem. 2017, 8, 2115–2122. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Sheltami, R.M.; Ahmad, I.; Abdullah, I.; Dufresne, A. Cellulose nanocrystal: A promising toughening agent for unsaturated polyester nanocomposite. Polymer 2015, 56, 346–357. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, G.-H.; Ha, C.-S. Polyimide/amine-functionalized cellulose nanocrystal nanocomposite films. Mater. Today Commun. 2017, 13, 275–281. [Google Scholar] [CrossRef]
- Yue, L.; Maiorana, A.; Khelifa, F.; Patel, A.; Raquez, J.-M.; Bonnaud, L.; Gross, R.; Dubois, P.; Manas-Zloczower, I. Surface-modified cellulose nanocrystals for biobased epoxy nanocomposites. Polymer 2018, 134, 155–162. [Google Scholar] [CrossRef]
- Myoung, S.H.; Im, S.S.; Kim, S.H. Non-isothermal crystallization behavior of PLA/acetylated cellulose nanocrystal/silica nanocomposites. Polym. Int. 2016, 65, 115–124. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Zhang, Y. Structure and properties of surface-acetylated cellulose nanocrystal/poly(butylene adipate-co-terephthalate) composites. Polym. Bull. 2016, 73, 2073–2085. [Google Scholar] [CrossRef]
- Parize, D.D.S.; de Oliveira, J.E.; Williams, T.; Wood, D.; Avena-Bustillos, R.J.; Klamczynski, A.P.; Glenn, G.M.; Marconcini, J.M.; Mattoso, L.H.C. Solution blow spun nanocomposites of poly(lactic acid)/cellulose nanocrystals from Eucalyptus kraft pulp. Carbohydr. Polym. 2017, 174, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, R.; Inoue, Y.; Shirakawa, M.; Miyashita, Y.; Nishio, Y. Cellulose alkyl ester/poly(ε-caprolactone) blends: Characterization of miscibility and crystallization behavior. Cellulose 2008, 15, 1–16. [Google Scholar] [CrossRef]
- Fox, J.D.; Capadona, J.R.; Marasco, P.D.; Rowan, S.J. Bioinspired water-enhanced mechanical gradient nanocomposite films that mimic the architecture and properties of the squid beak. J. Am. Chem. Soc. 2013, 135, 5167–5174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Karimkhani, V.; Makowski, B.T.; Samaranayake, G.; Rowan, S.J. Nanoemulsions and nanolatexes stabilized by hydrophobically functionalized cellulose nanocrystals. Macromolecules 2017, 50, 6032–6042. [Google Scholar] [CrossRef]
- Tan, C.; Peng, J.; Lin, W.; Xing, Y.; Xu, K.; Wu, J.; Chen, M. Role of surface modification and mechanical orientation on property enhancement of cellulose nanocrystals/polymer nanocomposites. Eur. Polym. J. 2015, 62, 186–197. [Google Scholar] [CrossRef]
- Eichhorn, S.; Dufresne, A.; Aranguren, M.; Marcovich, N.; Capadona, J.; Rowan, S.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Wu, G.-M.; Liu, G.-F.; Chen, J.; Kong, Z.-W. Preparation and properties of thermoset composite films from two-component waterborne polyurethane with low loading level nanofibrillated cellulose. Prog. Org. Coat. 2017, 106, 170–176. [Google Scholar] [CrossRef]
- Kobe, R.; Yoshitani, K.; Teramoto, Y. Fabrication of elastic composite hydrogels using surface-modified cellulose nanofiber as a multifunctional crosslinker. J. Appl. Polym. Sci. 2016, 133, 42906. [Google Scholar] [CrossRef]
- Dang, X.; Cao, X.; Ke, L.; Ma, Y.; An, J.; Wang, F. Combination of cellulose nanofibers and chain-end-functionalized polyethylene and their applications in nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45387. [Google Scholar] [CrossRef]
- Ansari, F.; Galland, S.; Johansson, M.; Plummer, C.J.G.; Berglund, L.A. Cellulose nanofiber network for moisture stable, strong and ductile biocomposites and increased epoxy curing rate. Compos. Part A 2014, 63, 35–44. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Zhang, X.; Xiao, M.; Zhang, W.; Lu, C. Grafting of polyethylenimine onto cellulose nanofibers for interfacial enhancement in their epoxy nanocomposites. Carbohydr. Polym. 2017, 157, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Chitpong, N.; Husson, S.M. Polyacid functionalized cellulose nanofiber membranes for removal of heavy metals from impaired waters. J. Membr. Sci. 2017, 523, 418–429. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, K.; Lin, Z.; Wu, W. Surface molecular-imprinting engineering of novel cellulose nanofibril/conjugated polymer film sensors towards highly selective recognition and responsiveness of nitroaromatic vapors. Chem. Commun. 2013, 49, 9137–9139. [Google Scholar] [CrossRef] [PubMed]
- Bideau, B.; Cherpozat, L.; Loranger, E.; Daneault, C. Conductive nanocomposites based on TEMPO-oxidized cellulose and poly(N-3-aminopropylpyrrole-co-pyrrole). Ind. Crops Prod. 2016, 93, 136–141. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. Organic Chemistry; Oxford University Press: Oxford, UK, 2005; pp. 279–303. [Google Scholar]
- Hakalahti, M.; Mautner, A.; Johansson, L.-S.; Hänninen, T.; Setälä, H.; Kontturi, E.; Bismarck, A.; Tammelin, T. Direct interfacial modification of nanocellulose films for thermoresponsive membrane templates. ACS Appl. Mater. Interfaces 2016, 8, 2923–2927. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Biermann, C.J. Grafting of maleic anhydride copolymers onto cellulose acetate and methyl cellulose. J. Wood Chem. Technol. 1992, 12, 231–240. [Google Scholar] [CrossRef]
- Kobe, R.; Iwamoto, S.; Endo, T.; Yoshitani, K.; Teramoto, Y. Stretchable composite hydrogels incorporating modified cellulose nanofiber with dispersibility and polymerizability: Mechanical property control and nanofiber orientation. Polymer 2016, 97, 480–486. [Google Scholar] [CrossRef]
- Mulyadi, A.; Deng, Y. Surface modification of cellulose nanofibrils by maleated styrene block copolymer and their composite reinforcement application. Cellulose 2016, 23, 519–528. [Google Scholar] [CrossRef]
- Kiziltas, E.E.; Kiziltas, A.; Nazari, B.; Gardner, D.J.; Bousfield, D.W. Glycerine Treated Nanofibrillated Cellulose Composites. J. Nanomater. 2016, 2016, 18–25. [Google Scholar] [CrossRef]
- Okieimen, F.E. Preparation, characterization, and properties of cellulose-polyacrylamide graft copolymers. J. Appl. Polym. Sci. 2003, 89, 913–923. [Google Scholar] [CrossRef]
- Littunen, K.; Hippi, U.; Johansson, L.-S.; Österberg, M.; Tammelin, T.; Laine, J.; Seppälä, J. Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr. Polym. 2011, 84, 1039–1047. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Poly(acrylic acid-co-acrylamide)/cellulose nanofibrils nanocomposite hydrogels: Effects of CNFs content on the hydrogel properties. Cellulose 2016, 23, 3691–3701. [Google Scholar] [CrossRef]
- Carlmark, A.; Malmström, E. Atom transfer radical polymerization from cellulose fibers at ambient temperature. J. Am. Chem. Soc. 2002, 124, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Crandall, C.; Prautzsch, V.L.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Electrospun regenerated cellulose nanofiber membranes surface-Grafted with water-insoluble poly(HEMA) or water-soluble poly(AAS) chains via the ATRP method for ultrafiltration of water. ACS Appl. Mater. Interfaces 2017, 9, 4272–4278. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Celebioglu, A.; Uyar, T. Surface modification of electrospun cellulose acetate nanofibers via RAFT polymerization for DNA adsorption. Carbohydr. Polym. 2014, 113, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Fu, S.Y.; Meng, Q.J.; Lucia, L.A. New insights into the material chemistry of polycaprolactone-grafted cellulose nanofibrils/polyurethane nanocomposites. Cellulose 2016, 23, 2457–2473. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The buildrs of microfibrillated cellulose and cationic polyelectr-up of polyelectrolyte multilayeolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Utsel, S.; Malmström, E.E.; Carlmark, A.; Wagberg, L. Thermoresponsive nanocomposites from multilayers of nanofibrillated cellulose and specially designed N-isopropylacrylamide based polymers. Soft Matter 2010, 6, 342–352. [Google Scholar] [CrossRef]
- Larsson, E.; Sanchez, C.C.; Porsch, C.; Karabulut, E.; Wågberg, L.; Carlmark, A. Thermo-responsive nanofibrillated cellulose by polyelectrolyte adsorption. Eur. Polym. J. 2013, 49, 2689–2696. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindstrom, T. Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. [Google Scholar]
- Wei, J.; Chen, Y.; Liu, H.; Du, C.; Yu, H.; Ru, J.; Zhou, Z. Effect of surface charge content in the TEMPO-oxidized cellulose nanofibers on morphologies and properties of poly(N-isopropylacrylamide)-based composite hydrogels. Ind. Crops Prod. 2016, 92, 227–235. [Google Scholar] [CrossRef]
- Uddin, A.J.; Araki, J.; Gotoh, Y. Toward “strong” green nanocomposites: Polyvinyl alcohol reinforced with extremely oriented cellulose whiskers. Biomacromolecules 2011, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Endo, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibril/poly(vinyl alcohol) composite drawn fibers. Polymer 2013, 54, 935–941. [Google Scholar] [CrossRef]
- Chaabouni, O.; Boufi, S. Cellulose nanofibrils/polyvinyl acetate nanocomposite adhesives with improved mechanical properties. Carbohydr. Polym. 2017, 156, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, J.; Zhang, Q.; Lei, M.; He, J.; Xiao, A.; Ma, C.; Li, S.; Xiong, H. Well-aligned cellulose nanofiber-reinforced polyvinyl alcohol composite film: Mechanical and optical properties. Carbohydr. Polym. 2016, 140, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kiziltas, A.; Nazari, B.; Kiziltas, E.E.; Gardner, D.J.S.; Han, Y.; Rushing, T.S. Cellulose nanofiber-polyethylene nanocomposites modified by polyvinyl alcohol. J. Appl. Polym. Sci. 2016, 133, 42933. [Google Scholar] [CrossRef]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.-S. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Safwan, A.; Sanyang, M.L.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Thermal and dynamic mechanical properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 102, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Mechanical, morphological and structural properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 97, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, K.; Yano, H.; Tsujii, Y. Surface engineering of cellulose nanofiber by adsorption of diblock copolymer dispersant for green nanocomposite materials. ACS Appl. Mater. Interfaces 2016, 8, 24893–24900. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Benitez, A.J.; Lossada, F.; Merindol, R.; Walther, A. Bioinspired mechanical gradients in cellulose nanofibril/polymer nanopapers. Angew. Chem. Int. Ed. 2016, 55, 5966–5970. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.J.; Lossada, F.; Zhu, B.; Rudolph, T.; Walther, A. Understanding toughness in bioinspired cellulose nanofibril/polymer nanocomposites. Biomacromolecules 2016, 17, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Laya, M.; Méndez, J.A.; Delgado-Aguilar, M.; Bun, K.N.; Vilaseca, F. Strong and electrically conductive nanopaper from cellulose nanofibers and polypyrrole. Carbohydr. Polym. 2016, 152, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Bideau, B.; Bras, J.; Saini, S.; Daneault, C.; Loranger, E. Mechanical and antibacterial properties of a nanocellulose-polypyrrole multilayer composite. Mater. Sci. Eng. C 2016, 69, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Xu, L.; Zhang, L.; Ye, H.; Zhao, J.; Liu, Z.; Rong, J. Superior hybrid hydrogels of polyacrylamide enhanced by bacterial cellulose nanofiber clusters. Mater. Sci. Eng. C 2016, 67, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Napadensky, E.; Orlicki, J.A.; Snyder, J.F.; Chantawansri, T.L.; Kapllani, A. Cellulose nanofibrils and diblock copolymer complex: Micelle formation and enhanced dispersibility. ACS Sustain. Chem. Eng. 2017, 5, 1264–1271. [Google Scholar] [CrossRef]
- Qu, P.; Zhou, Y.; Zhang, X.; Yao, S.; Zhang, L. Surface modification of cellulose nanofibrils for poly(lactic acid) composite application. J. Appl. Polym. Sci. 2012, 125, 3084–3091. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Vuluga, Z.; Vitelaru, C.; Damian, C.M. The effect of cellulose nanofibers on the crystallinity and nanostructure of poly(lactic acid) composites. J Mater. Sci. 2016, 51, 9771–9791. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Chen, W.; Liu, Y.; Wang, Q.; Li, J.; Yu, H. Homogeneous dispersion of cellulose nanofibers in waterborne acrylic coatings with improved properties and unreduced transparency. ACS Sustain. Chem. Eng. 2016, 4, 3766–3772. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Sillard, C.; Hassager, O.; Daugaard, A.E.; Bras, J.; Szabo, P. A comparison of partially acetylated nanocellulose, nanocrystalline cellulose, and nanoclay as fillers for high-performance polylactide nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43257. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Song, P.; Lei, W.; Wu, Q.; Liu, L.; Yu, Y.; Wang, H. Fire-Resistant, strong, and green polymer nanocomposites based on poly(lactic acid) and core-shell nanofibrous flame retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Soman, S.; Chacko, A.S.; Prasad, V.S. Semi-interpenetrating network composites of poly(lactic acid) with cis-9-octadecenylamine modified cellulose-nanofibers from Areca catechu husk. Compos. Sci. Technol. 2017, 141, 65–73. [Google Scholar] [CrossRef]
- Sato, A.; Kabusaki, D.; Okumura, H.; Nakatani, T.; Nakatsubo, F.; Yano, H. Surface modification of cellulose nanofibers with alkenyl succinic anhydride for high-density polyethylene reinforcement. Composites Part A 2016, 83, 72–79. [Google Scholar] [CrossRef]
- Croitoru, C.; Patachia, S. Long-chain alkylimidazolium ionic liquid functionalization of cellulose nanofibers and their embedding in HDPE matrix. Int. J. Polym. Sci. 2016, 2016, 7432528. [Google Scholar] [CrossRef]
- Sakakibara, K.; Moriki, Y.; Yano, H.; Tsujii, Y. Strategy for the Improvement of the Mechanical Properties of Cellulose Nanofiber-Reinforced High-Density Polyethylene Nanocomposites Using Diblock Copolymer Dispersants. ACS Appl. Mater. Interfaces 2017, 9, 44079–44087. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhao, Y.; Kuga, S.; Wu, M.; Huang, Y. A versatile method for producing functionalized cellulose nanofibers and their application. Nanoscale 2016, 8, 3753–3759. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Sain, S.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ray, D. Surface treatment of cellulose fibers with methylmethacrylate for enhanced properties of in situ polymerized PMMA/cellulose composites. J. Appl. Polym. Sci. 2014, 131, 39808. [Google Scholar] [CrossRef]
- Peng, Y.; Gallegos, S.A.; Gardner, D.J.; Han, Y.; Cai, Z. Maleic anhydride polypropylene modified cellulose nanofibril polypropylene nanocomposites with enhanced impact strength. Polym. Compos. 2016, 37, 782–793. [Google Scholar] [CrossRef]
- Wang, L.; Gardner, D.J.; Bousfield, D.W. Cellulose nanofibril-reinforced polypropylene composites for material extrusion: Rheological properties. Polym. Eng. Sci. 2017, 58, 793–801. [Google Scholar] [CrossRef]
- Ferrer, A.; Hoeger, I.C.; Lu, X.; Rojas, O.J. Reinforcement of polypropylene with lignocellulose nanofibrils and compatibilization with biobased polymers. J. Appl. Polym. Sci. 2016, 133, 43854. [Google Scholar] [CrossRef]
- Iwamoto, S.; Yamamoto, S.; Lee, S.-H.; Endo, T. Solid-state shear pulverization as effective treatment for dispersing lignocellulose nanofibers in polypropylene composites. Cellulose 2014, 21, 1573–1580. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013, 32, 383–394. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.-B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 2014, 30, 9327–9335. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Facile route to transparent, strong, and thermally stable nanocellulose/polymer nanocomposites from an aqueous pickering emulsion. Biomacromolecules 2017, 18, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, N.; Qazvini, N.T.; Deng, Y. Surfactant free Pickering emulsion polymerization of styrene in W/O/W system using cellulose nanofibrils. Eur. Polym. J. 2015, 64, 179–188. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Hemmati, M.; Deng, Y.; Qazvini, N.T. Water expandable polystyrene containing cellulose nanofibrils: Expansion behavior and morphology. Chem. Eng. Sci. 2016, 156, 56–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Wang, B.; Sui, X.; Zhong, Y.; Zhang, L.; Mao, Z.; Xu, H. Cellulose nanofibril-reinforced biodegradable polymer composites obtained via a Pickering emulsion approach. Cellulose 2017, 24, 3313–3322. [Google Scholar] [CrossRef]





















| Surface Modifier for CNC | Compatible Polymer | Key Improvements in the Nanocomposite | Ref. |
|---|---|---|---|
 methyl(triphenyl) phosphonium bromide (MePh3PBr) | Epoxy/PS |
| Fox et al. [168] |
 hexadecyltrimethyl ammonium bromide (QS) |  PP |
| Nagalakshmaiah et al. [170] |
| –do– | UV-curable acrylic coating |
| Kaboorani et al. [171] |
 phenylbutyl isocyanate |  poly(butylene adipate-co-terephthalate) (PBAT) |
| Morelli et al. [180] |
 acetic anhydride |  PLA |
| Myoung et al. [188] |
| –do– | Amine-cured epoxy resin |
| Abraham et al. [146] |
| –do– |  PBAT |
| Zhang et al. [189] |
 maleic anhydride (MA) |  PLA |
| Parize et al. [190] |
 polyethylene glycol (PEG) |  HDPE |
| Inai et al. [172] |
| –do– |  LDPE |
| Azouz et al. [173] |
| –do– |  Polybutylene succinate (PBS) |
| Ludueña et al. [177] |
 allyl amine (Reaction with COOH–CNC) |  Polyvinyl acetate (PVAc) |
| Fox et al. [192] |
 NCO-terminated Coumarin |  Poly(epichlorohydrin-co-ethylene oxide) |
| Biyani et al. [181] |
 NCO-terminated diarylbibenzofuranone (DABBF) | DABBF-based crosslinked polymer |
| Imato et al. [184] |
 NCO-terminated UPy- |  UPy-functionalized poly(ethylene-co-butylene) |
| Biyani et al. [182] |
| –do– | LDPE LLDPE SBS EO-EPI |
| Natte-rodt et al. [183] |
 n-octadecyl isocyanate | PLA/NR blend |
| Bitinis et al. [179] |
 L-Lactide | –do– |
| –do– |
 N-(β-aminoethyl)-ɣ-aminopropyltrimethoxysilane (APTES) |  Unsaturated polyester resin (UPR) |
| Kargarz-adeh et al. [185] |
 (3-aminopropyl) trimethoxysilane | Epoxy resin |
| Yue et al. [187] |
 (3-aminopropyl) triethoxysilane | Polyimide |
| Lee et al. [186] |
| Surface Modifier for CNF | Compatible Polymer | Key Improvements in the Nanocomposite | Reference |
|---|---|---|---|
| CH3COOH acetic acid |  polylactic acid (PLA) |
| Trifol et al. [240] |
 cis-9-octadecenylamine (OA) | –do– |
| Soman et al. [242] |
 3-methacryloxypropyl trimethoxysilane (MEMO) | –do– |
| Qu et al. [237] |
 3-aminopropyl triethoxysilane (APS) | –do– |
| Frone et al. [238] |
| –do– | Waterborne acrylic resin/polyester blend |
| Tan et al. [239] |
 PLMA-b-PHEMA |  HDPE |
| Sakakibara et al. [230] |
 PDCPMA-b-PHEMA |  HDPE |
| Sakakibara et al. [245] |
 alkenylsuccinic anhydride |  HDPE |
| Sato et al. [243] |
 1-Hexyl-3-methyl imidazolium tetrafluoroborate (HMIMBF4) |  HDPE | – | Croitoru et al. [244] |
 n-dodecylsuccinic anhydride |  PE |
| Huang et al. [246] |
 maleic anhydride-grafted polypropylene (MAPP) |  PP |
| Peng et al. [248] |
| –do– | –do– |
| Wang et al. [249] |
 methyl methacrylate (MMA) |  PMMA |
| Banerjee et al. [247] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517. https://doi.org/10.3390/polym10050517
Chakrabarty A, Teramoto Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers. 2018; 10(5):517. https://doi.org/10.3390/polym10050517
Chicago/Turabian StyleChakrabarty, Arindam, and Yoshikuni Teramoto. 2018. "Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them" Polymers 10, no. 5: 517. https://doi.org/10.3390/polym10050517