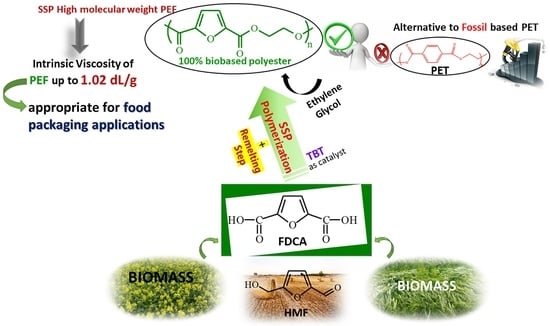
Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 2,5-Dimethylfuran-Dicarboxylate(DMFD)
2.3. Polyester Synthesis
2.4. Solid-State Polycondensation
2.5. Polyester Characterization
2.5.1. Intrinsic Viscosity Measurement
2.5.2. Molecular Weight
2.5.3. End-Group Analysis
2.5.4. Differential Scanning Calorimetry (DSC)
3. Modeling the PEF SSP Kinetics
3.1. Reaction Mechanism




3.2. Simplified Mathematical Model
- -
- All kinetic rate constants are considered independent of polymer chain length (only end-group reactivity is considered).
- -
- Backward reactions in Equations (3) and (4) are eliminated, due to the fast removal of the water and ethylene glycol, produced in the reaction mixture, caused by the application of high vacuum (beneath 3 and 4 Pa).
- -
- Due to the performance of the polycondensation at relatively low temperatures (i.e., 190–205 °C), no side reactions for the formation of acetaldehyde or thermal degradation are considered (Equations (5) and (6) are eliminated).
- -
- Diffusional limitations on account of desorbing volatile species are neglected.
- -
4. Results and Discussion
4.1. Kinetic Study of the Solid-State Polymerization of PEF
4.2. Thermal Analysis of Solid-State Polymerization PEF Polyester Samples
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass, Volume I–Results of Screening for Potential Candidates from Sugars and Synthesis Gas; (DOE/GO-102004–1992); National Renewable Energy Laboratory, US Department of Energy: Springfield, VA, USA, 2004.
- Thiyagarajan, S.; Genuino, H.C.; van der Waal, J.C.; De Jong, E.; Weckhuysen, B.M.; Van Haveren, J.; Bruijnincx, P.C.; Van Es, D.S. A Facile solid-phase route to renewable aromatic chemicals from biobased furanics. Angew. Chem. Int. Ed. 2016, 55, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Antonietti, M. Redefining biorefinery: The search for unconventional building blocks for materials. Chem. Soc. Rev. 2015, 44, 5821–5835. [Google Scholar] [CrossRef] [PubMed]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furandicarboxylate) for packaging applications: Excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Soares, M.J.; Dannecker, P.-K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester. J. Anal. Appl. Pyrolysis 2017, 126, 357–370. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast crystallization and melting behavior of a long-spaced aliphatic furandicarboxylate bio-based polyester, the poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New poly(pentylene furanoate) and poly(heptylene furanoate) sustainable polyesters from diols with odd methylene groups. Mater. Lett. 2016, 178, 64–67. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Exarhopoulos, S.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Sustainable, eco-friendly polyesters synthesized from renewable resources: Preparation and thermal characteristics of poly(dimethyl-propylene furanoate). Polym. Chem. 2015, 6, 8284–8296. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.-H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furan dicarboxylate), a Biobased Alternative to PBT: Synthesis, Physical Properties, and Crystal Structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and Characterization of Bio-Based Polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexane dimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Carlos Morales-Huerta, J.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic acid (FDCA), A versatile building block for a very interesting class of polyesters. ACS Symp. Ser. 2012, 1105, 1–13. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellence. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films. Catalysts 2017, 7, 318. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.J.I.; Knoop, R.; Frissen, A.E.; Van Haveren, J.; Van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957–1966. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and Polymorphism of Poly(ethylene furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- PEF: Game-Changing Plastic. Available online: https://www.avantium.com/yxy/products-applications/ (accessed on 26 March 2015).
- Steinborn-Rogulska, I.; Rokicki, G. Solid-state polycondensation (SSP) as a method to obtain high molecular weight polymers. Part II. Synthesis of polylactide and polyglycolide via SSP. Polimery 2013, 58, 85–92. [Google Scholar] [CrossRef]
- Vouyiouka, S.N.; Karakatsani, E.K.; Papaspyrides, C.D. Solid state polymerization. Prog. Polym. Sci. 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Vouyiouka, S.N. Solid State Polymerization, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–294. ISBN 9780470084182. [Google Scholar]
- Zhang, J.; Shen, X.-J.; Zhang, J.; Feng, L.-F.; Wang, J.-J. Experimental and modeling study of the solid state polymerization of poly(ethylene terephthalate) over a wide range of temperatures and particle sizes. J. Appl. Polym. Sci. 2013, 127, 3814–3822. [Google Scholar] [CrossRef]
- Li, L.-J.; Duan, R.-T.; Zhang, J.-B.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. Phosphorus-Containing Poly(ethylene terephthalate): Solid-State Polymerization and Its Sequential Distribution. Ind. Eng. Chem. Res. 2013, 52, 5326–5333. [Google Scholar] [CrossRef]
- Gantillon, B.; Spitz, R.; McKenna, T.F. The Solid State Postcondensation of PET, 1: A Review of the Physical and Chemical Processes Taking Place in the Solid State. Macromol. Mater. Eng. 2004, 289, 88–105. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Kokkalas, D.E.; Bikiaris, D.N. Solid-state polycondensation of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. II. J. Appl. Polym. Sci. 1995, 56, 405–410. [Google Scholar] [CrossRef]
- Bikiaris, D.; Karavelidis, V.; Karayannidis, G. A New Approach to Prepare Poly(ethylene terephthalate)/Silica Nanocomposites with Increased Molecular Weight and Fully Adjustable Branching or Crosslinking by SSP. Macromol. Rapid Commun. 2006, 27, 1199–1205. [Google Scholar] [CrossRef]
- Achilias, D.S.; Bikiaris, D.N.; Karavelidis, V.; Karayannidis, G.P. Effect of silica nanoparticles on solid state polymerization of poly(ethylene terephthalate). Eur. Polym. J. 2008, 44, 3096–3107. [Google Scholar] [CrossRef]
- Van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sbirrazzuoli, N. Biaxial Orientation of Poly(ethylene 2,5-furandicarboxylate): An Explorative Study. Macromol. Mater. Eng. 2018, 303, 1700507. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Physical aging in amorphous poly(ethylene furanoate): Enthalpic recovery, density, and oxygen transport considerations. J. Polym. Sci. Part B 2015, 53, 389–399. [Google Scholar] [CrossRef]
- Guigo, N.; van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Modelling the non-isothermal crystallization of polymers: Application to poly(ethylene 2,5-furandicarboxylate). Thermochim. Acta 2017, 650, 66–75. [Google Scholar] [CrossRef]
- Codou, A.; Moncel, M.; Van Berkel, J.G.; Guigo, N.; Sbirrazzuoli, N. Glass transition dynamics and cooperativity length of poly(ethylene 2,5-furandicarboxylate) compared to poly(ethylene terephthalate). Phys. Chem. Chem. Phys. 2016, 18, 16647–16658. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 1: Equilibrium sorption. Polymer 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sipos, L.; Wang, B.; Dam, M.A.; Sbirrazzuoli, N. Isothermal Crystallization Kinetics of Poly(Ethylene 2,5-Furandicarboxylate). Macromol. Mater. Eng. 2014, 300, 466–474. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; Van Berkel, J.; De Jong, E.; Sbirrazzuoli, N. Non-isothermal Crystallization Kinetics of Biobased Poly(ethylene 2,5-furandicarboxylate) Synthesized via the Direct Esterification Process. Macromol. Chem. Phys. 2014, 215, 2065–2074. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Stoclet, G.; Gobius Du Sart, G.; Yeniad, B.; De Vos, S.; Lefebvre, J.M. Isothermal crystallization and structural characterization of poly(ethylene-2,5-furanoate). Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Maini, L.; Gigli, M.; Gazzano, M.; Lotti, N.; Bikiaris, D.N.; Papageorgiou, G.Z. Structural investigation of poly(ethylene furanoate) polymorphs. Polymers 2018, 10, 296. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: Thermal behavior comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly(ethylene furanoate). J. Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, S.; Haernvall, K.; Scaini, D.; Ghazaryan, G.; Zumstein, M.T.; Sander, M.; Pellis, A.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene furanoate) thin films of various crystallinities. Green Chem. 2017, 19, 5381–5384. [Google Scholar] [CrossRef]
- Stoclet, G.; Lefebvre, J.M.; Yeniad, B.; Gobius du Sart, G.; De Vos, S. On the strain-induced structural evolution of Poly(ethylene-2,5-furanoate) upon uniaxial stretching: An in-situ SAXS-WAXS study. Polymer 2018, 134, 227–241. [Google Scholar] [CrossRef]
- Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and structural response of in situ prepared biobased poly(ethylene 2,5-furan dicarboxylate) nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A Series of Furan-Aromatic Polyesters Synthesized via Direct Esterification Method Based on Renewable Resources. J. Polym. Sci. Part A 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Roo, J.D.; Storti, G.; Morbidelli, M. Diffusion (DOSY) 1H NMR as an Alternative Method for Molecular Weight Determination of Poly(ethylene furanoate) (PEF) Polyesters. Macromol. Chem. Phys. 2017, 218, 1600436. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and Characterization of Poly(2,5-furan dicarboxylate)s Based on a Variety of Diols. J. Polym. Sci. Part A 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Gruter, G.-J.M.; Sipos, L.; Dam, M.A. Accelerating Research into Bio-Based FDCA-Polyesters by Using Small Scale Parallel Film Reactors. Comb. Chem. High Throughput Screen. 2012, 15, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State Polymerization of Poly(ethylene furanoate) Biobased Polyester, I: Effect of Catalyst Type on Molecular Weight Increase. Polymers 2017, 9, 607. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Tsanaktsis, V.; Nikolaidis, N.; Kostoglou, M.; Papageorgiou, G.Z.; Lambropoulou, D.A.; Bikiaris, D.A. Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation. Polym. Chem. 2017, 8, 6895–6908. [Google Scholar] [CrossRef]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.-V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid State Polymerization of Poly(Ethylene Furanoate) and Its Nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302, 1700012. [Google Scholar] [CrossRef]
- Sipos, L. A Process for Preparing a Polymer having a 2,5-Furandicarboxylate Moiety within the Polymer Backbone and Such (Co)Polymers, (Furanix Technologies B.V.). W.O. Patent 2010/077133 A1, 8 July 2010. [Google Scholar]
- Knoop, R.J.I.; Vogelzang, W.; Van Haveren, J.; Van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.-D.; Nam, B.-U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5150. [Google Scholar] [CrossRef]
- Duh, B. Reaction Kinetics for Solid-State Polymerization of Poly(ethylene terephthalate). J. Appl. Polym. Sci. 2001, 81, 1748–1761. [Google Scholar] [CrossRef]
- Weissmann, D. PET Use in Blow Molded Rigid Packaging. In Applied Plastics Engineering Handbook, 1st ed.; Kutz, M., Ed.; William Andrew: Waltham, MA, USA, 2011; pp. 603–623. ISBN 978-143773514-7. [Google Scholar]
- Gupta, V.B.; Bashir, Z. PET Fibers, Films, and Bottles. In Handbook of Thermoplastic Polyesters: Homopolymers, Copolymers, Blends and Composites, 1st ed.; Fakirov, S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 317–361. ISBN 9783527301133. [Google Scholar]
- Ros-Chumillas, M.; Belissario, Y.; Iguaz, A.; López, A. Quality and shelf life of orange juice aseptically packaged in PET bottles. J. Food Eng. 2007, 79, 234–242. [Google Scholar] [CrossRef]
- Berkowitz, S. Viscosity–molecular weight relationships for poly(ethylene terephthalate) in hexafluorois opropanol–pentafluorophenol using SEC–LALLS. J. Appl. Polym. Sci. 1984, 29, 4353–4361. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Kokkalas, D.E.; Bikiaris, D.N. Solid-state polycondensation of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. J. Appl. Polym. Sci. 1993, 50, 2135–2142. [Google Scholar] [CrossRef]
- Pohl, H.A. Determination of carboxyl end groups in a polyester, polyethylene terephthalate. Anal. Chem. 1954, 26, 1614–1616. [Google Scholar] [CrossRef]
- Ravindranath, K.; Mashelkar, R.A. Modeling of Poly(ethylene Terephthalate) Reactors. I. A Semibatch Ester Interchange Reactor. J. Appl. Polym. Sci. 1981, 26, 3179–3204. [Google Scholar] [CrossRef]
- Mallon, F.K.; Ray, W.H. Modeling of solid-state polycondensation. II. Reactor design issues. J. Appl. Polym. Sci. 1998, 69, 1775–1788. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S.; Sikkema, D.J.; Lemstra, P.J. Solid-state polymerization of PET: Influence of nitrogen sweep and high vacuum. Polymer 2003, 44, 4085–4096. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S. Solvent assisted post-polymerization of PET. Polymer 2005, 46, 5447–5455. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S.; Giliopoulos, D.J.; Karayannidis, G.P. Effect of activated carbon black nanoparticles on solid state polymerization of poly(ethylene terephthalate). Eur. Polym. J. 2006, 42, 3190–3201. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karandrea, E.; Triantafyllidis, K.S.; Ladavos, A.; Bikiaris, D.N. Effect of organoclays type on solid-state polymerization (SSP) of poly(ethylene terephthalate): Experimental and modelling. Eur. Polym. J. 2015, 63, 156–167. [Google Scholar] [CrossRef]
- Achilias, D.S.; Gerakis, K.; Giliopoulos, D.J.; Triantafyllidis, K.S.; Bikiaris, D.N. Effect of high surface area mesoporous silica fillers (MCF and SBA-15) on solid state polymerization of PET. Eur. Polym. J. 2016, 81, 347–364. [Google Scholar] [CrossRef]
- Kaushik, A.; Gupta, S.K. A molecular model for solid-state polymerization of nylon 6. J. Appl. Polym. Sci. 1992, 45, 507–520. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Gupta, S.K. Molecular Model for Solid-state Polymerization of Nylon 6. II. An Improved Model. J. Appl. Polym. Sci. 1994, 53, 85–103. [Google Scholar] [CrossRef]
- Gaymans, R.J.; Amirtharaj, J.; Kamp, H. Nylon 6 Polymerization in the Solid State. J. Appl. Polym. Sci. 1982, 27, 2513–2526. [Google Scholar] [CrossRef]
- Li, L.F.; Huang, N.X.; Tang, Z.L.; Hagen, R. Reaction kinetics and simulation for the solid-state polycondensation of nylon 6. Macromol. Theory Simul. 2001, 10, 507–517. [Google Scholar] [CrossRef]
- Duh, B. Semiempirical rate equation for solid state polymerization of poly(ethylene terephthalate). J. Appl. Polym. Sci. 2002, 84, 857–870. [Google Scholar] [CrossRef]
- Wu, D.; Chen, F.; Li, R.; Shi, Y. Reaction kinetics and simulations for solid-state polymerization of poly(ethylene terephthalate). Macromolecules 1997, 30, 6737–6742. [Google Scholar] [CrossRef]









| Temperature (°C) | SSP time (h) | PEF/TBT.1 | PEF/TBT.2 | PEF/TBT.3 |
|---|---|---|---|---|
| 0 | 4400 (24) | 7400 (40) | 15,000 (81) | |
| 190 | 1 | 8000 (44) | 7700 (42) | 15,800 (86) |
| 2 | 9300 (51) | 8300 (45) | 18,600 (101) | |
| 3.5 | 10,000 (54) | 10,000 (54) | 20,700 (112) | |
| 5 | 10,300 (56) | 10,600 (58) | 21,600 (117) | |
| 200 | 1 | 8300 (45) | 8300 (45) | 17,400 (90) |
| 2 | 9600 (52) | 9000 (49) | 19,800 (108) | |
| 3.5 | 10,600 (58) | 10,300 (56) | 22,900 (124) | |
| 5 | 11,300 (62) | 11,000 (59) | 26,100 (142) | |
| 205 | 1 | 10,300 (56) | 9600 (52) | 20,300 (110) |
| 2 | 11,300 (62) | 11,700 (63) | 24,200 (132) | |
| 3.5 | 11,900 (63) | 12,400 (67) | 31,400 (171) | |
| 5 | 12,400 (67) | 12,700 (69) | 33,900 (184) |
| Sample | Temperature (°C) | k1·(kg/meq)·h−1 | k2·(kg/meq)·h−1 | [OH]i (meq/kg) | [COOH]i (meq/kg) |
|---|---|---|---|---|---|
| PEF/TBT.1 | 190 | 39 × 10−4 | 51 × 10−4 | 157 | 9.5 |
| 200 | 50 × 10−4 | 64 × 10−4 | 150 | 7.5 | |
| 205 | 59 × 10−4 | 71 × 10−4 | 138 | 6.0 | |
| PEF/TBT.2 | 190 | 2.0 × 10−4 | 22 × 10−4 | 60 | 11.5 |
| 200 | 3.2 × 10−4 | 31 × 10−4 | 58 | 11.5 | |
| 205 | 6.6 × 10−4 | 72 × 10−4 | 52 | 11.5 | |
| PEF/TBT.3 | 190 | 15 × 10−4 | 14 × 10−4 | 31 | 9.0 |
| 200 | 25 × 10−4 | 21 × 10−4 | 26 | 9.0 | |
| 205 | 36 × 10−4 | 37 × 10−4 | 13 | 9.0 |
| Sample | E1 (kJ/mol) | R2 | E2 (kJ/mol) | R2 |
|---|---|---|---|---|
| PEF/TBT.1 | 50.0 ± 4.0 | 0.996 | 40.7 ± 0.6 | 0.999 |
| PEF/TBT.2 | 137.4 ± 11.4 | 0.950 | 133.1 ± 15.1 | 0.907 |
| PEF/TBT.3 | 105.3 ± 9.7 | 0.979 | 112.5 ± 24.1 | 0.895 |
| SSP temperature (°C) | SSP time (h) | PEF/TBT.1 | PEF/TBT.2 | PEF/TBT.3 |
|---|---|---|---|---|
| 0 | 23 | 28.8 | 2.7 | |
| 190 | 1 | 37.7 | 38.1 | 29.6 |
| 2 | 43.8 | 38.9 | 31 | |
| 3.5 | 44 | 40.9 | 34.5 | |
| 5 | 47.3 | 46.2 | 36.7 | |
| 200 | 1 | 43 | 39.2 | 20.2 |
| 2 | 45.5 | 40 | 26.7 | |
| 3.5 | 46.3 | 40.5 | 30.3 | |
| 5 | 49.3 | 48.2 | 31.5 | |
| 205 | 1 | 46.3 | 43.3 | 27.8 |
| 2 | 48.4 | 44.2 | 28.4 | |
| 3.5 | 50.8 | 44.8 | 31.5 | |
| 5 | 54.4 | 45.4 | 37.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications. Polymers 2018, 10, 471. https://doi.org/10.3390/polym10050471
Kasmi N, Papageorgiou GZ, Achilias DS, Bikiaris DN. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications. Polymers. 2018; 10(5):471. https://doi.org/10.3390/polym10050471
Chicago/Turabian StyleKasmi, Nejib, George Z. Papageorgiou, Dimitris S. Achilias, and Dimitrios N. Bikiaris. 2018. "Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications" Polymers 10, no. 5: 471. https://doi.org/10.3390/polym10050471