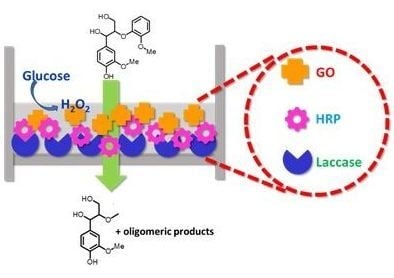
Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immobilization of Enzymes in Functionalized Membranes
2.2. Performance of the Enzyme-Immobilized Membranes towards Degradation of the Lignin Model Compound
3. Results and Discussions
3.1. Characterizations of the Functionalized PVDF Membranes
3.2. Reactivity of the Membrane Bioreactors towards GGE Degradation/Modification
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tokarev, I.; Minko, S. Multiresponsive, hierarchically structured membranes: New, challenging, biomimetic materials for biosensors, controlled release, biochemical gates, and nanoreactors. Adv. Mater. 2009, 21, 241–247. [Google Scholar] [CrossRef]
- Cho, Y.; Lim, J.; Char, K. Layer-by-layer assembled stimuli-responsive nanoporous membranes. Soft Matter 2012, 8, 10271–10278. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sanders, J.P. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.M.; Batista-Viera, F. Immobilization of enzymes. In Immobilization of Enzymes and Cells; Guisan, J.M., Ed.; Humana Press: New York, NY, USA, 2006; pp. 15–30. [Google Scholar]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Wang, B.; Tan, J.; Zhu, L.; Li, L. Immobilized multienzymatic systems for catalysis of cascade reactions. Process Biochem. 2016, 51, 1193–1203. [Google Scholar] [CrossRef]
- Jia, F.; Narasimhan, B.; Mallapragada, S. Materials-based strategies for multi-enzyme immobilization and co-localization: A review. Biotechnol. Bioeng. 2014, 111, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Rosini, E.; Allegretti, C.; Melis, R.; Cerioli, L.; Conti, G.; Pollegioni, L.; D’Arrigo, P. Cascade enzymatic cleavage of the β-O-4 linkage in a lignin model compound. Catal. Sci. Technol. 2016, 6, 2195–2205. [Google Scholar] [CrossRef]
- Xu, P.; Yu, B.; Li, F.L.; Cai, X.F.; Ma, C.Q. Microbial degradation of sulfur, nitrogen and oxygen heterocycles. Trends Microbiol. 2006, 14, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Dec, J.; Bollag, J.-M. Use of enzymes in bioremediation. In Pesticide Biotransformation in Plants and Microorganisms; American Chemical Society: Washington, DC, USA, 2000; pp. 182–193. [Google Scholar]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin—Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef] [Green Version]
- Gasser, C.A.; Hommes, G.; Schaffer, A.; Corvini, P.F. Multi-catalysis reactions: New prospects and challenges of biotechnology to valorize lignin. Appl. Microbiol. Biotechnol. 2012, 95, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Hoberg, J.O.; Dimmel, D.R. Heteropolyacid catalyzed oxidation of lignin and lignin models to benzoquinones. J. Wood Chem. Technol. 2000, 20, 19–41. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Liu, G.; Dotzauer, D.M.; Bruening, M.L. Ion-exchange membranes prepared using layer-by-layer polyelectrolyte deposition. J. Membr. Sci. 2010, 354, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Dotzauer, D.M.; Dai, J.; Sun, L.; Bruening, M.L. Catalytic membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006, 6, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Datta, S.; Gui, M.; Coker, E.L.; Huggins, F.E.; Daunert, S.; Bachas, L.; Bhattacharyya, D. Reactive nanostructured membranes for water purification. Proc. Natl. Acad. Sci. USA 2011, 108, 8577–8582. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Noda, A.; Nakanishi, K.; Matsuno, R.; Kamikubo, T. Thermal stability of immobilized glucoamylase entrapped in polyacrylamide gels and bound to SP-Sephadex C–50. Agric. Biol. Chem. 1980, 44, 2047–2054. [Google Scholar]
- Kim, Y.D.; Dordick, J.S.; Clark, D.S. Siloxane-based biocatalytic films and paints for use as reactive coatings. Biotechnol. Bioeng. 2001, 72, 475–482. [Google Scholar] [CrossRef]
- Cao, L. Introduction: Immobilized enzymes: Past, present and prospects. In Carrier-Bound Immobilized Enzymes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–52. [Google Scholar]
- Bhattacharyya, D.; Ganapathi, S.; Vishwanath, S.; Summers, M.; Butterfield, D.A. Immobilized enzyme reactions on beads and membranes. In Biofunctional Membranes; Butterfield, D.A., Ed.; Springer: Boston, MA, USA, 1996; pp. 117–129. [Google Scholar]
- Jochems, P.; Satyawali, Y.; Diels, L.; Dejonghe, W. Enzyme immobilization on/in polymeric membranes: Status, challenges and perspectives in biocatalytic membrane reactors (BMRs). Green Chem. 2011, 13, 1609–1623. [Google Scholar] [CrossRef]
- Bhunia, A.; Durani, S.; Wangikar, P.P. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Moreira, T.M.; Moldes-Diz, Y.; Feijoo, S.; Eibes, G.; Lema, M.J.; Feijoo, G. Formulation of laccase nanobiocatalysts based on ionic and covalent interactions for the enhanced oxidation of phenolic compounds. Appl. Sci. 2017, 7, 851. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. BioResources 2009, 4, 1694–1717. [Google Scholar]
- Roy-Arcand, L.; Archibald, F.S. Direct dechlorination of chlorophenolic compounds by laccases from trametes (coriolus) versicolor. Enzym. Microb. Technol. 1991, 13, 194–203. [Google Scholar] [CrossRef]
- Jolivalt, C.; Brenon, S.; Caminade, E.; Mougin, C.; Pontié, M. Immobilization of laccase from trametes versicolor on a modified PVDF microfiltration membrane: Characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membr. Sci. 2000, 180, 103–113. [Google Scholar] [CrossRef]
- Xu, R.; Chi, C.; Li, F.; Zhang, B. Laccase–polyacrylonitrile nanofibrous membrane: Highly immobilized, stable, reusable, and efficacious for 2,4,6-trichlorophenol removal. ACS Appl. Mater. Interfaces 2013, 5, 12554–12560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stemple, B.; Kumar, M.; Wei, N. Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol a and sulfamethoxazole. Environ. Sci. Technol. 2016, 50, 8799–8808. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Perazzini, R.; Saladino, R. Oxidative functionalisation of lignin by layer-by-layer immobilised laccases and laccase microcapsules. Appl. Catal. A Gen. 2010, 372, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Moeder, M.; Martin, C.; Koeller, G. Degradation of hydroxylated compounds using laccase and horseradish peroxidase immobilized on microporous polypropylene hollow fiber membranes. J. Membr. Sci. 2004, 245, 183–190. [Google Scholar] [CrossRef]
- Nicell, J.A.; Bewtra, J.K.; Taylor, K.E.; Biswas, N.; St. Pierre, C. Enzyme catalyzed polymerization and precipitation of aromatic compounds from wastewater. Water Sci. Technol. 1992, 25, 157–164. [Google Scholar]
- Nicell, J.A.; Bewtra, J.K.; Biswas, N.; Taylor, E. Reactor development for peroxidase catalyzed polymerization and precipitation of phenols from wastewater. Water Res. 1993, 27, 1629–1639. [Google Scholar] [CrossRef]
- He, F.; Luo, B.; Yuan, S.; Liang, B.; Choong, C.; Pehkonen, S.O. PVDF film tethered with rgd-click-poly(glycidyl methacrylate) brushes by combination of direct surface-initiated atrp and click chemistry for improved cytocompatibility. RSC Adv. 2014, 4, 105–117. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; van Bronswijk, W. In situ FTIR-ATR examination of poly(acrylic acid) adsorbed onto hematite at low ph. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Penu, R.; Vasilescu, I.; Eremia, S.A.V.; Gatea, F.; Radu, G.-L.; Litescu, S.-C. Development of a nanocomposite system and its application in biosensors construction. Cent. Eur. J. Chem. 2013, 11, 968–978. [Google Scholar] [CrossRef]
- Sarma, R.; Islam, M.S.; Miller, A.F.; Bhattacharyya, D. Layer-by-layer-assembled laccase enzyme on stimuli-responsive membranes for chloro-organics degradation. ACS Appl. Mater. Interfaces 2017, 9, 14858–14867. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Papp, J.K.; Colburn, A.S.; Meeks, N.D.; Weaver, B.; Wilf, I.; Bhattacharyya, D. Engineered iron/iron oxide functionalized membranes for selenium and other toxic metal removal from power plant scrubber water. J. Membr. Sci. 2015, 488, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Qian, X.; Wickramasinghe, S.R. Responsive membranes for advanced separations. Curr. Opin. Chem. Eng. 2015, 8, 98–104. [Google Scholar] [CrossRef]
- Datta, S.; Cecil, C.; Bhattacharyya, D. Functionalized membranes by layer-by-layer assembly of polyelectrolytes and in situ polymerization of acrylic acid for applications in enzymatic catalysis. Ind. Eng. Chem. Res. 2008, 47, 4586–4597. [Google Scholar] [CrossRef]
- Xiao, L.; Davenport, D.M.; Ormsbee, L.; Bhattacharyya, D. Polymerization and functionalization of membrane pores for water related applications. Ind. Eng. Chem. Res. 2015, 54, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Brissos, V.; Tavares, D.; Sousa, A.C.; Robalo, M.P.; Martins, L.O. Engineering a bacterial DyP-type peroxidase for enhanced oxidation of lignin-related phenolics at alkaline pH. ACS Catal. 2017, 7, 3454–3465. [Google Scholar] [CrossRef]
- Lončar, N.; Colpa, D.I.; Fraaije, M.W. Exploring the biocatalytic potential of a DyP-type peroxidase by profiling the substrate acceptance of thermobifida fusca DyP peroxidase. Tetrahedron 2016, 72, 7276–7281. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Velasco-Lozano, S.; Guisán, J.M.; López-Gallego, F. Oxidation of phenolic compounds catalyzed by immobilized multi-enzyme systems with integrated hydrogen peroxide production. Green Chem. 2014, 16, 303–311. [Google Scholar] [CrossRef]









| Reactors | Of the Total GGE Conversion Product | Total GGE Conversion | ||
|---|---|---|---|---|
| A (m/z 251) | B (m/z 661) | C (m/z 979) | ||
| Laccase | 99% | 0.8% | - | 73% |
| HRP | 43% | 9.9% | 2.4% | 57% |
| Multienzyme | 62% | 0.7% | 0.4% | 95% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarma, R.; Islam, M.S.; Running, M.P.; Bhattacharyya, D. Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound. Polymers 2018, 10, 463. https://doi.org/10.3390/polym10040463
Sarma R, Islam MS, Running MP, Bhattacharyya D. Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound. Polymers. 2018; 10(4):463. https://doi.org/10.3390/polym10040463
Chicago/Turabian StyleSarma, Rupam, Md. Saiful Islam, Mark P. Running, and Dibakar Bhattacharyya. 2018. "Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound" Polymers 10, no. 4: 463. https://doi.org/10.3390/polym10040463