Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Gelatin and Collagen-Gelatin Microspheres
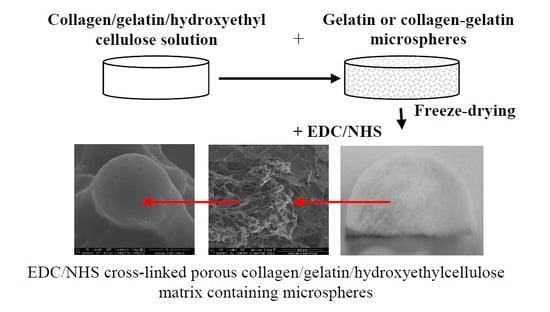
2.3. Production of Collagen-Gelatin Matrices Incorporating Gel and Col-Gel Microspheres
2.4. Characterization of the Composites
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Attenuated Total Reflection Infrared Spectroscopy (ATR-FTIR)
2.4.3. Porosity and Density Measurement
2.4.4. Swelling Tests
2.4.5. Dissolution of Matrices
2.4.6. Mechanical Properties
2.5. Incorporation of Calendula Officinalis Extract into the Matrices
2.6. Loading Capacity of Matrices
2.7. In Vitro Release
3. Results and Discussion
3.1. Structure of Prepared Materials, Porosity and Density
3.2. ATR-FTIR Spectroscopy Results
3.3. Swelling Tests
3.4. Dissolution of Matrices
3.5. Mechanical Properties
3.6. Loading Capacity of Matrices
3.7. In Vitro Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joseph, M.; Trinh, H.M.; Mitra, A.K. Peptide and Protein-Based Therapeutic Agents. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A., Cholkar, K., Mandal, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–167. ISBN 978-0-323-42978-8. [Google Scholar]
- Wang, Y.; Burgess, D.J. Microspheres Technologies. In Long Acting Injections and Implants; Wright, J., Burgess, D., Eds.; Springer: New York, NY, USA, 2012; pp. 167–194. ISBN 978-1-4614-0553-5. [Google Scholar]
- Del Mercato, L.L.; Passione, L.G.; Izzo, D.; Rinaldi, R.; Sannino, A.; Gervaso, F. Design and characterization of microcapsules-integrated collagen matrixes as multifunctional three-dimensional scaffolds for soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 62, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Xu, T.; Lu, X.; Zhi, W.; Weng, J. Porous hydroxyapatite scaffolds containing dual microspheres based on poly(lactide-co-glycolide) and chitosan for bone regeneration. Mater. Lett. 2017, 188, 387–391. [Google Scholar] [CrossRef]
- Wang, C.; Luo, W.; Li, P.; Li, S.; Yang, Z.; Hu, Z.; Liu, Y.; Ao, N. Preparation and evaluation of chitosan/alginate porous microspheres/Bletilla striata polysaccharide composite hemostatic sponges. Carbohydr. Polym. 2017, 174, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Grabska, S. Preparation and characterization of 3D collagen materials with magnetic properties. Polym. Test. 2017, 62, 382–391. [Google Scholar] [CrossRef]
- Jiménez, R.A.; Millán, D.; Suesca, E.; Sosnik, A.; Fontanilla, M.R. Controlled release of an extract of Calendula officinalis flowers from a system based on the incorporation of gelatin-collagen microparticles into collagen I scaffolds: Design and in vitro performance. Drug Deliv. Transl. Res. 2015, 5, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.E.; Kwon, I.C.; Ahn, H.J.; Lee, S.H.; Cho, H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 2004, 25, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, J.; Sionkowska, A. Effects of different crosslinking methods on the properties of collagen–calcium phosphate composite materials. Int. J. Biol. Macromol. 2015, 74, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.K.; Mitra, A.K. Kinetics of in vitro release of a model nucleoside deoxyuridine from crosslinked insoluble collagen and collagen–gelatin microspheres. Int. J. Pharm. 1999, 193, 113–122. [Google Scholar] [CrossRef]
- Tylingo, R.; Gorczyca, G.; Mania, S.; Szweda, P.; Milewski, S. Preparation and characterization of porous scaffolds from chitosan-collagen-gelatin composite. React. Funct. Polym. 2016, 103, 131–140. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Habermehl, J.; Vesentini, S.; Mantovani, D. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials 2015, 26, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Usha, R.; Sreeram, K.J.; Rajaram, A. Stabilization of collagen with EDC/NHS in the presence of l-lysine: A comprehensive study. Colloids Surf. B Biointerfaces 2012, 90, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Charulatha, V.; Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003, 24, 759–767. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Chipara, A.C.; Narayanan, N.T.; Anumary, A.; Sruthi, R.; Thanikaivelan, P.; Vajtai, R.; Mani, S.A.; Ajayan, P.M. Three-Dimensional Porous Sponges from Collagen Bio-Wastes. ACS Appl. Mater. Interfaces 2016, 8, 14836–14844. [Google Scholar] [CrossRef] [PubMed]
- Yahyouche, A.; Zhidao, X.; Czernuszka, J.T.; Clover, A.J. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomater. 2011, 7, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, N.; Qiu, J.; Jiang, H.; Zhao, H.; Wang, G.; Boughton, R.I.; Wang, Y.; Liu, H. Carbodiimide crosslinked collagen from porcine dermal matrix for high-strength tissue engineering scaffold. Int. J. Biol. Macromol. 2013, 61, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.J.; Weiss-Bilka, H.E.; Meagher, M.J.; Liu, Y.; Gargac, J.A.; Niebur, G.L.; Wagner, D.R.; Roeder, R.K. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater. 2015, 17, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tsung, J.; Burgess, J.D. Biodegradable Polymers in Drug Delivery Systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, A.R., Rathbone, M.J., Eds.; Springer: New York, NY, USA, 2012; pp. 107–123. ISBN 978-1-4614-0880-2. [Google Scholar]
- Gullapalli, R.P.; Mazzitelli, C.L. Gelatin and Non-Gelatin Capsule Dosage Forms. J. Pharm. Sci. 2017, 106, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Adhirajan, N.; Shanmugasundaram, N.; Shanmuganathan, S.; Babu, M. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur. J. Pharm. Sci. 2009, 36, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lu, L.; Gunasekaran, S. Gold nanoparticle-based thermal history indicator for monitoring low-temperature storage. Microchim. Acta 2015, 182, 1305–1311. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Bhowmik, S.; Islam, J.M.M.; Debnath, T.; Miah, M.Y.; Bhattacharjee, S.; Khan, M.A. Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications. Polymers 2017, 9, 222. [Google Scholar] [CrossRef]
- Defail, A.J.; Edington, H.D.; Matthews, S.; Lee, W.C.; Marra, K.G. Controlled release of bioactive doxorubicin from microspheres embedded within gelatin scaffolds. J. Biomed. Mater. Res. A 2006, 79, 954–962. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.F.; Abu-Serieb, M.M.; Hassanc, M.A.; Elnoubyd, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Chahal, S.; Hussain, F.S.J.; Kumar, A.; Bahari, M.S.; Rasad, A.; Yusoff, M.M. Fabrication, characterization and in vitro biocompatibility of electrospun hydroxyethyl cellulose/poly(vinyl) alcohol nanofibrous composite biomaterial for bone tissue engineering. Chem. Eng. Sci. 2016, 144, 17–29. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Ding, C.; Li, Y.; Wang, Y.; Li, J.; Sun, Y.; Lin, Y.; Sun, W.; Luo, C. Highly selective adsorption of hydroquinone by hydroxyethyl cellulose functionalized with magnetic/ionic liquid. Int. J. Biol. Macromol. 2018, 107, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Andrabi, K.I.; John, R. Calendula officinalis—An Important Medicinal Plant with Potential Biological Properties. Proc. Indian Natl. Sci. Acad. 2017, 83, 769–787. [Google Scholar]
- Butnariu, M.; Coradini, C.Z. Evaluation of Biologically Active Compounds from Calendula officinalis Flowers using Spectrophotometry. Chem Cent J. 2012, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, J.; Sionkowska, A.; Skopińska-Wiśniewska, J.; Piechowicz, K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 8, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Suzuki, S.; Tabata, Y.; Ikada, Y.; Nishimura, Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 2000, 21, 489–499. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–58. [Google Scholar]
- Rössler, B.; Kreuter, J.; Scherer, D. Collagen microparticles: Preparation and properties. J. Microencapsul. 1995, 12, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Anumary, A.; Thanikaivelan, P.; Ashokkumar, M.; Kumar, R.; Sehgal, P.K.; Chandrasekaran, B. Synthesis and Characterization of Hybrid Biodegradable Films from Bovine Hide Collagen and Cellulose Derivatives for Biomedical Applications. Soft Mater. 2013, 11, 181–194. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K.; Grabska, S.; Pokrywczyńska, M.; Kloskowski, T.; Drewa, T. 3D composites based on the blends of chitosan and collagen with the addition of hyaluronic acid. Int. J. Biol. Macromol. 2016, 89, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.P.; Wang, Y.J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.B.; Wang, L.Y.; Ji, P.H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 95, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Kok, S.H.; Bian, Z.X.; Lam, K.H.; Tang, J.C.; Lee, K.K.; Gambari, R.; Chui, C.H. d-Glucose as a modifying agent in gelatin/collagen matrix and reservoir nanoparticles for Calendula officinalis delivery. Colloids Surf. B Biointerfaces 2014, 117, 277–283. [Google Scholar] [CrossRef] [PubMed]










| Sample | (%) | d (g/mL) |
|---|---|---|
| col/gel/hec | 86.0 ± 2.79 | 0.012 ± 0.0019 |
| col/gel/hec (gel) | 79.3 ± 1.28 | 0.014 ± 0.0020 |
| col/gel/hec (col-gel) | 81.0 ± 3.21 | 0.017 ± 0.0017 |
| Sample | Position of the Band (cm-1) | ||||||
|---|---|---|---|---|---|---|---|
| Amide A | Amide B | C–H | Amide I | Amide II | Amide III | C–O | |
| col | 3419 | 3077 | 2924 | 1657 | 1553 | 1241 | 1057 |
| gel | 3291 | 3067 | 2925 | 1623 | 1520 | 1225 | 1063 |
| col/gel/hec | 3294 | 3064 | 2922 | 1631 | 1542 | 1226 | 1054 |
| col/gel/hec (gel) | 3296 | 3066 | 2919 | 1627 | 1535 | 1232 | 1056 |
| col/gel/hec (col-gel) | 3292 | 3072 | 2920 | 1638 | 1532 | 1231 | 1050 |
| Sample | Ec (kPa) ± SD | |
|---|---|---|
| Dry Samples | Samples Soaked in Phosphate Buffer Saline (PBS) | |
| col/gel/hec | 7.02 ± 0.09 | 0.314 ± 0.10 |
| col/gel/hec (gel) | 6.97 ± 1.00 | 0.411 ± 0.08 |
| col/gel/hec (col-gel) | 6.44 ± 0.23 | 0.382 ± 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlowska, J.; Stachowiak, N.; Sionkowska, A. Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation. Polymers 2018, 10, 456. https://doi.org/10.3390/polym10040456
Kozlowska J, Stachowiak N, Sionkowska A. Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation. Polymers. 2018; 10(4):456. https://doi.org/10.3390/polym10040456
Chicago/Turabian StyleKozlowska, Justyna, Natalia Stachowiak, and Alina Sionkowska. 2018. "Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation" Polymers 10, no. 4: 456. https://doi.org/10.3390/polym10040456