Angular Photochromic LC Composite Film for an Anti-Counterfeiting Label
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Preparation of the Samples
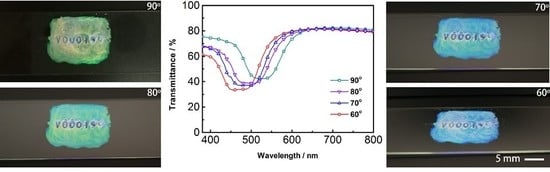
3. Results and Discussion
3.1. Mesomorphic and Optical Properties of the Cholesteric Liquid Crystal Materials
3.2. Dependence of the Polymer Dispersed Network on the Optical and Mechanical Properties
3.3. Preparation of Angular Photochromic Films with a Coexistent Polymer Dispersed Network
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, Y.T.; Chi, S. Compact, reliable asymmetric optical configuration for cost-effective fabrication of multiplex dot matrix hologram in anti-counterfeiting applications. Optik 2003, 114, 161. [Google Scholar] [CrossRef]
- Huang, S.; Wu, J.K. Optical Watermarking for Printed Document Authentication. IEEE Trans. Inf. Forensics Secur. 2007, 2, 164. [Google Scholar] [CrossRef]
- Hida, M.; Mitsui, T.; Minami, Y. Forensic investigation of counterfeit coins. Forensic Sci. Int. 1997, 89, 21. [Google Scholar] [CrossRef]
- Nakayama, K.; Ohtsubo, J. Optical security device providing fingerprint and designed pattern indicator using fingerprint texture in liquid crystal. Opt. Eng. 2012, 51, 040506. [Google Scholar] [CrossRef]
- Li, W.S.; Shen, Y.; Chen, Z.J.; Cui, Q.; Li, S.S.; Chen, L.J. Demonstration of patterned polymer-stabilized cholesteric liquid crystal textures for anti-counterfeiting two-dimensional barcodes. Appl. Opt. 2017, 56, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Ohtsubo, J. Optical security devices using nonuniform schlieren texture of UV-curable nematic liquid crystal. Appl. Opt. 2016, 55, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Vartak, S.; Eakin, J.N.; Faris, S.M. Surface anchoring effects on spectral broadening of cholesteric liquid crystal films. J. Appl. Phys. 2008, 104, 023108. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Shan, Y.; Gong, L.; Chen, J.; Li, S.; Chen, L. Magnetic Nanoparticle-Assisted Tunable Optical Patterns from Spherical Cholesteric Liquid Crystal Bragg Reflectors. Nanomaterials 2017, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Tondiglia, V.P.; Godman, N.P.; Middletonab, C.M.; White, T.J. Blue-shifting tuning of the selective reflection of polymer stabilized cholesteric liquid crystals. Soft Matter 2017, 13, 5842–5848. [Google Scholar]
- Li, Y.; Yang, T.; Yu, T.; Zheng, L.; Liao, K. Blue-shifting tuning of the selective reflection of polymer stabilized cholesteric liquid crystals. J. Mater. Chem. 2011, 21, 10844–10851. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Q.; Li, Z.; Fan, G.; Xiong, D.; Su, Y.; Zhang, J.; Zhang, D. Enhanced Mechanical Properties of Graphene (Reduced Graphene Oxide)/Aluminum Composites with a Bioinspired Nanolaminated Structure. Nano Lett. 2015, 15, 8077–8083. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ning, N.; Wei, Z.; Zhan, L.; Tian, M.; Zou, H.; Mi, J. Evolution of conductive network and properties of nanorod/polymer composite under tensile strain. Polymer 2014, 55, 3178–3185. [Google Scholar] [CrossRef]
- Jang, J.; Bouveret, B.; Suhr, J.; Gibson, R.F. Combined numerical/experimental investigation of particle diameter and interphase effects on coefficient of thermal expansion and young's modulus of SiO2. Polym. Composites 2012, 33, 1415–1423. [Google Scholar] [CrossRef]
- Huber, J.; Griesshaber, E.; Nindiyasari, F.; Schmahl, W.W.; Ziegler, A. Functionalization of biomineral reinforcement in crustacean cuticle: Calcite orientation in the partes incisivae of the mandibles of Porcellio scaber and the supralittoral species Tylos europaeus. J. Struct. Biol. 2015, 190, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.D.K.; Hadal, R.; Duncan, S.J. Surface damage behavior during scratch deformation of mineral reinforced polymer composites. Acta Mater. 2004, 52, 4363–4376. [Google Scholar] [CrossRef]
- Dasari, A.; Rohrmann, J.; Misra, R.D.K. On the scratch deformation of micrometric wollastonite reinforced polypropylene composites. Mater. Sci. Eng. A 2004, 364, 357–369. [Google Scholar] [CrossRef]
- Chen, S.; Tong, X.; He, H.; Ma, M.; Shi, Y.; Wang, X. Body Temperature Controlled Optical and Thermal Information Storage Light Scattering Display with Fluorescence Effect and High Mechanical Strength. ACS Appl. Mater. Interfaces 2017, 9, 11924. [Google Scholar] [CrossRef] [PubMed]
- Rafiquzzaman, M.; Islam, M.; Rahman, H.; Talukdar, S.; Hasan, N. Mechanical property evaluation of glass–jute fiber reinforced polymer composites. Polym. Adv. Technol. 2016, 27, 1308–1316. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Kong, N.; Barrow, C.J.; Yang, W. RAFT controlled synthesis of graphene/polymer hydrogel with enhanced mechanical property for pH-controlled drug release. Eur. Polym. J. 2014, 50, 9–17. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, W.; Sun, J.; Zhang, H.; Wang, Z.; Wang, L.; Yang, D.; Zhang, L.; Yang, H. A novel soft matter composite material for energysaving smart windows: from preparation to device application. J. Mater. Chem. A 2015, 3, 10738. [Google Scholar] [CrossRef]
- Song, P.; Gao, Y.; Wang, F.; Zhang, L.; Xie, H.; Yang, Z.; Yang, H. Middle Jurassic oceanic island igneous rocks of the Raohe accretionary complex, northeastern China: Petrogenesis and tectonic implications. Liquid Crystals 2015, 42, 390–396. [Google Scholar] [CrossRef]
- Gao, Y.; Song, P.; Zhang, T.; Yao, W.; Ding, H.; Xiao, J.; Zhu, S.; Cao, H.; Yang, H. Effects of a triethylamine catalyst on curing time and electro-optical properties of PDLC films. RSC Adv. 2013, 3, 23533–23538. [Google Scholar] [CrossRef]
- Yu, H.; Dong, C.; Zhou, W.; Kobayashi, T.; Yang, H. Wrinkled liquid-crystalline microparticle-enhanced photoresponse of PDLC-like films by coupling with mechanical stretching. Small 2011, 7, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Kashima, M.; Cao, H.; Meng, Q.; Liu, H.; Wang, D.; Li, F.; Yang, H. The influence of crosslinking agents on the morphology and electro-optical performances of PDLC films. J. Appl. Polym. Sci. 2010, 117, 3434–3440. [Google Scholar] [CrossRef]
- Liang, X.; Guo, S.; Chen, M.; Li, C.; Wang, Q.; Zou, C.; Zhang, C.; Zhang, L.; Guo, S.; Yang, H. A temperature and electric field-responsive flexible smart film with full broadband optical modulation. Mater. Horiz. 2017, 5, 878–884. [Google Scholar] [CrossRef]
- Guo, S.; Liang, X.; Zhan, C.; Chen, M.; Shen, C.; Zhang, L.; Yuan, X.; He, B.; Yang, H. Preparation of a Thermally Light-Transmittance-Controllable Film from a Coexistent System of Polymer-Dispersed and Polymer-Stabilized Liquid Crystals. ACS Appl. Mater. Interfaces 2017, 9, 2942–2947. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.H.; Cao, H.; Zhao, D.Y.; Hu, W.; He, W.L.; Yuan, X.T.; Xiao, J.M.; Zhang, H.Q.; Yang, H. Chiral nematic liquid crystals with helix inversion from (R)-1,1′-binaphthyl and cholesteryl ester moieties. Liq. Cryst. 2011, 38, 9–15. [Google Scholar] [CrossRef]
- Yao, W.H.; Gao, Y.Z.; Yuan, X.; He, B.F.; Yu, H.F.; Zhang, L.Y.; Zhi, Z.H.; He, W.L.; Yang, Z.; Yang, H.; et al. Synthesis and self-assembly behaviours of side-chain smectic thiol–ene polymers based on the polysiloxane backbone. J. Mater. Chem. C 2016, 4, 1425. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24, 6260. [Google Scholar] [CrossRef] [PubMed]







| ChLC | Contents | Phase Transition b/°C | Selective Reflection Wavelength/nm |
|---|---|---|---|
| S1 | SLC1717/S811 3/1 a | Cr −15.5 Ch 83.1 Iso | 668 |
| S2 | C6M/S811 3/1 a | Cr 77.6 Ch 106.8 Iso | 675 |
| S3 | 3HG2080 | g 59.0 Ch 215.5 Iso | 525 |
| Sample | UA a/IPDI b/TTEG c/SLC1717/C6M/S811/3HG2080 aWeight Ratio | Polymerization Method |
|---|---|---|
| A1 | 20/0/0/60/0/20/0 | Photo-polymerization |
| A2 | 20/0/0/0/60/20/0 | Photo-polymerization |
| A3 | 20/0/0/0/0/0/80 | Photo-polymerization |
| B1 | 0/10/10/60/0/20/0 | Thermo-polymerization |
| B2 | 0/10/10/0/60/20/0 | Thermo-polymerization |
| B3 | 0/10/10/0/0/0/80 | Thermo-polymerization |
| C1 | 100/0/0/0/0/0/0 | Photo-polymerization |
| C2 | 25/37.5/37.5/0/0/0/0 | Photo- and thermo-polymerization |
| C3 | 50/25/25/0/0/0/0 | Photo- and thermo-polymerization |
| C4 | 75/12.5/12.5/0/0/0/0 | Photo- and thermo-polymerization |
| C5 | 0/50/50/0/0/0/0 | Thermo-polymerization |
| D1 | 10/5/5/60/0/20/0 | Photo- and thermo-polymerization |
| D2 | 10/5/5/0/0/0/80 | Photo- and thermo-polymerization |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Yao, W.; Sun, J.; Li, K.; Zhang, L. Angular Photochromic LC Composite Film for an Anti-Counterfeiting Label. Polymers 2018, 10, 453. https://doi.org/10.3390/polym10040453
Gao Y, Yao W, Sun J, Li K, Zhang L. Angular Photochromic LC Composite Film for an Anti-Counterfeiting Label. Polymers. 2018; 10(4):453. https://doi.org/10.3390/polym10040453
Chicago/Turabian StyleGao, Yanzi, Wenhuan Yao, Jian Sun, Kexuan Li, and Lanying Zhang. 2018. "Angular Photochromic LC Composite Film for an Anti-Counterfeiting Label" Polymers 10, no. 4: 453. https://doi.org/10.3390/polym10040453