Renewable, Eugenol—Modified Polystyrene Layer for Liquid Crystal Orientation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
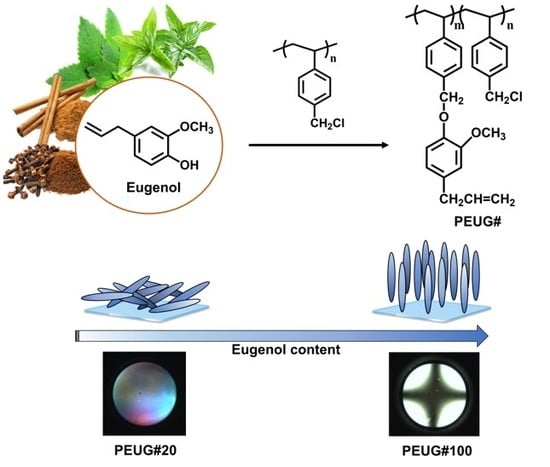
2.2. Synthesis of Eugenol Modified Polystyrenes
2.3. Film Preparation and LC Cell Assembly
2.4. Instrumentation
3. Results and Discussion
3.1. Synthesis and Characterization of Eugenol Modified Polystyrene
3.2. Transmittance of Eugenol Modified Polystyrene Film
3.3. LC Orientation Behavior of the LC Cells Fabricated with Eugenol Modified Polystyrene Films
3.4. Surface Properties of Eugenol Modified Polystyrene Films
3.5. Reliability and Electro-Optical Performance of the LC Cells Fabricated with Eugenol Modified Polystyrene Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chandrasekhar, S. Liquid Crystals, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; ISBN 0-521-41747-3. [Google Scholar]
- Meyer, R.B. Effects of electric and magnetic fields on the structure of cholesteric liquid crystals. Appl. Phys. Lett. 1968, 12, 281–282. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E. Materials: Introduction and Applications, 1st ed.; John Wiley & Sons: West Sussex, UK, 2017; pp. 242–246. ISBN 978-1-119-28100-9. [Google Scholar]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef]
- Mori, H.; Itoh, Y.; Nishiura, Y.; Nakamura, T.; Shinagawa, T. Performance of a novel optical compensation film based on negative birefringence of discotic compound for wide-viewing-angle twisted-nematic liquid-crystal displays. Jpn. J. Appl. Phys. 1997, 36, 143–147. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: An emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S. Liquid crystals in photovoltaics: A new generation of organic photovoltaics. Polym. J. 2017, 49, 85–111. [Google Scholar] [CrossRef]
- Stöhr, J.; Samant, M.G.; Cossy-Favre, A.; Diaz, J.; Momoi, Y.; Odahara, S.; Nagata, T. Microscopic origin of liquid crystal alignment on rubbed polymer surfaces. Macromolecules 1998, 31, 1942–1946. [Google Scholar] [CrossRef]
- Takatoh, K.; Sakamoto, M.; Hasegawa, R.; Koden, M.; Itoh, N.; Hasegawa, M. Alignment Technology and Applications of Liquid Crystal Devices; CRC Press: Boca Raton, FL, USA, 2005; pp. 7–8. ISBN 978-07-484-0902-0. [Google Scholar]
- Ichimura, K. Photoalignment of liquid-crystal systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, J.; Samant, M.G. Liquid crystal alignment by rubbed polymer surfaces: A microscopic bond orientation model. J. Electron. Spectrosc. Relat. Phenom. 1999, 98, 189–207. [Google Scholar] [CrossRef]
- Schadt, M. Liquid crystal materials and liquid crystal displays. Annu. Rev. Mater. Sci. 1997, 27, 305–379. [Google Scholar] [CrossRef]
- Latansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L. Polyimides: Fundamentals and Applications; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1996; pp. 806–807. ISBN 978-0824794668. [Google Scholar]
- Feller, M.B.; Chen, W.; Shen, T.R. Investigation of surface-induced alignment of liquid-crystal molecules by optical second-harmonic generatio. Phys. Rev. A 1991, 43, 6778–6792. [Google Scholar] [CrossRef] [PubMed]
- Van Aerle, J.N.A.; Tol, A.J.W. Molecular orientation in rubbed polyimide alignment layers used for liquid-crystal displays. Macromolecules 1994, 27, 6520–6526. [Google Scholar] [CrossRef]
- Lee, K.W.; Peak, S.-H.; Lien, A.; Durning, C.; Fukuro, H. Microscopic molecular reorientation of alignment layer polymer surfaces induced by rubbing and its effects on LC pretilt angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar] [CrossRef]
- Weiss, K.; Wöll, C.; Böhm, E.; Fiebranz, B.; Forstmann, G.; Peng, B.; Scheumann, V.; Johannsmann, D. Molecular orientation at rubbed polyimide surfaces determined with X-ray absorption spectroscopy: Relevance for liquid crystal alignment. Macromolecules 1998, 31, 1930–1936. [Google Scholar] [CrossRef]
- Meister, R.; Jerome, B. The conformation of a rubbed polyimide. Macromolecules 1999, 32, 480–486. [Google Scholar] [CrossRef]
- Ge, J.J.; Li, C.Y.; Xue, G.; Mann, I.K.; Zhang, D.; Wang, S.-Y.; Harris, F.W.; Cheng, S.Z.D.; Hong, S.-C.; Zhuang, X. Rubbing-induced molecular reorientation on an alignment surface of an aromatic polyimide containing cyanobiphenyl side chains. J. Am. Chem. Soc. 2001, 123, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Oh-e, M.; Shen, Y.R. Rubbed polyimide surface studied by sum-frequency vibrational spectroscopy. Macromolecules 2001, 34, 9125–9129. [Google Scholar] [CrossRef]
- Vaughn, K.E.; Sousa, M.; Kang, D.; Rosenblatt, C. Continuous control of liquid crystal pretilt angle from homeotropic to planar. Appl. Phys. Lett. 2007, 90, 194102. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Ree, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.-B. Liquid-crystal alignment on the rubbed film surface of semi-flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Liq. Cryst. 2000, 349, 279–282. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.W.; Ha, J.D.; Oh, J.M.; Yi, M.H. Synthesis and characterization of novel polyimides with 1-octadecyl side chains for liquid crystal alignment layers. Polym. Adv. Technol. 2007, 18, 226–234. [Google Scholar] [CrossRef]
- Lee, S.W.; Chae, B.; Lee, B.; Choi, W.; Kim, S.B.; Kim, S.I. Rubbing-induced surface morphology and polymer segmental reorientations of a model brush polyimide and interactions with liquid crystals at the surface. Chem. Mater. 2003, 15, 3105–3112. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, G.J.; Chi, J.H.; Zin, W.-C.; Jung, J.C.; Hahm, S.G.; Ree, M.; Chang, T. Synthesis, characterization and liquid-crystal-aligning properties of novel aromatic polypyromellitimides bearing (n-alkyloxy) biphenyloxy side chains. Polymer 2006, 47, 6606–6621. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.S.; Kang, D.; Lee, J.-C. Liquid crystal alignment property of n-alkylthiomethyl- or n-alkylsulfonylmethyl-substituted polystyrenes. Polym. Adv. Technol. 2009, 20, 878–886. [Google Scholar] [CrossRef]
- Kang, H.; Kim, T.-H.; Kang, D.; Lee, J.-C. 4-Alkylphenoxymethyl-substituted polystyrenes for liquid crystal alignment layers. Macromol. Chem. Phys. 2009, 210, 926–935. [Google Scholar] [CrossRef]
- Szabadics, J.; Erdelyi, L. Pre-and postsynaptic effects of eugenol and related compounds on Helix pomatia L. neurons. Acta Biol. Hung. 2000, 51, 265–273. [Google Scholar] [PubMed]
- Prakash, P.; Gupta, N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J. Physiol. Pharmacol. 2005, 49, 125–131. [Google Scholar] [PubMed]
- Tokuoka, Y.; Uchiyama, H.; Abe, M.; Ogino, K. Solubilization of synthetic perfumes by nonionic surfactants. J. Colloid Interface Sci. 1992, 152, 402–409. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- El-Baroty, G.S.; El-Baky, H.A.; Farag, R.; Saleh, M.A. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr. J. Biochem. Res. 2010, 4, 167–174. [Google Scholar]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Balszyk, M.; Holley, A. Interaction of monolaurin, eugenol and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int. J. Food Microbiol. 1998, 39, 175–183. [Google Scholar] [CrossRef]
- Karapinar, M.; Aktuǧ, Ş.E. Inhibition of foodborne pathogens by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol. 1987, 4, 161–166. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C.I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Kim, O.H.; Park, S.W.; Park, H.D. Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol. 2004, 21, 105–110. [Google Scholar] [CrossRef]
- Schmalz, G.; Arenholt-Bindslev, D. Biocompatibility of Dental Materials, 1st ed.; Springer: Heidelberg, Germany, 2009; ISBN 978-3-540-77782-3. [Google Scholar]
- Grespan, R.; Paludo, M.; de Paula Lemos, H.; Barbosa, C.P.; Bersani-Amado, C.A.; de Oliveira Dalalio, M.M.; Cuman, R.K.N. Anti-arthritic effect of eugenol on collagen-induced arthritis experimental model. Biol. Pharm. Bull. 2012, 35, 1818–1820. [Google Scholar] [CrossRef] [PubMed]
- Garkal, D.; Taralkar, S.P.; Kulkarni, P.; Jagtap, S.; Nagawade, A. Kinetic model for extraction of eugenol from leaves of Ocimum Sanctum Linn (Tulsi). Int. J. Pharm. Appl. 2012, 3, 267–270. [Google Scholar]
- Ryu, D.Y.; Shin, K.; Drockenmuller, E.; Hawker, C.J.; Russell, T.P. A generalized approach to the modification of solid surfaces. Science 2005, 308, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Whitesides, G.M. Depth sensitivity of wetting: Monolayers of ω-mercapto ethers on gold. J. Am. Chem. Soc. 1988, 110, 5897–5898. [Google Scholar] [CrossRef]
- Ju, C.; Kim, T.; Kang, H. Liquid crystal alignment behaviors on capsaicin substituted polystyrene films. RSC Adv. 2017, 7, 41376–41383. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Gedde, U. Polymer Physics; Chapman and Hall: London, UK, 1995; pp. 78–79. ISBN 978-94-011-0543-9. [Google Scholar]
- Brostow, W. Realiability and prediction of long term performance of polymer-based materials. Pure Appl. Chem. 2009, 81, 417–432. [Google Scholar] [CrossRef]
- Hayes, R.A. The relationship between glass temperature, molar cohesion, and polymer structure. J. Appl. Polym. Sci. 1961, 15, 318–321. [Google Scholar] [CrossRef]
- Wesslin, B.; Lenz, R.W.; MacKnight, W.J.; Karaz, F.E. Glass transition temperatures of poly(ethyl α-chloroacrylates). Macromolecules 1971, 4, 24–26. [Google Scholar] [CrossRef]
- Lee, J.-C.; Litt, M.H.; Rogers, C.E. Oxyalkylene polymers with alkylsulfonylmethyl side chains: Gas barrier properties. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 75–83. [Google Scholar] [CrossRef]
- Lee, J.-B.; Lee, H.-K.; Park, J.-C.; Kim, Y.-B. The structural effect on the pretilt angle of alignment materials with alkylcyclohexylbenzene as a side chain in polyimides. Mol. Cryst. Liq. Cryst. 2005, 439, 161–172. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.J.; Hahm, S.G.; Lee, T.J.; Lee, B.; Chae, B.; Kim, S.B.; Jung, J.C.; Zin, W.C.; Sohn, B.H. Role of the n-alkyl end of bristles governing liquid crystal alignment at rubbed films of brush polymer rods. Macromolecules 2005, 38, 4331–4338. [Google Scholar] [CrossRef]
- Paek, S.H.; Durning, C.J.; Lee, K.W.; Lien, A. A mechanistic picture of the effects of rubbing on polyimide surfaces and liquid crystal pretilt angles. J. Appl. Phys. 1998, 83, 1270–1280. [Google Scholar] [CrossRef]
- Ban, B.S.; Kim, Y.B. Surface energy and pretilt angle on rubbed polyimide surfaces. J. Appl. Polym. Sci. 1999, 74, 267–271. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, C.Y.; Lin, C.J.; Pan, R.P.; Lin, S.S.; Lee, C.D.; Kou, C.S. Mechanism in determining pretilt angle of liquid crystals aligned on fluorinated copolymer films. J. Phys. D Appl. Phys. 2009, 42, 155303. [Google Scholar] [CrossRef]
- MacDonald, B.A.; Rollins, K.; Mackerron, D.; Rakos, K.; Eveson, R.; Hashimoto, K.; Rustin, B. Engineered films for display technologies. In Flexible Flat Panel Displays; Crawford, G.P., Ed.; John Wiley & Sons: West Sussex, UK, 2005; pp. 11–33. ISBN 978-0-470-87048-8. [Google Scholar]







| Polymer Designation | Eugenol [mol %] | Degree of Substitution [%] | Mn 1 | Mw/Mn 1 |
|---|---|---|---|---|
| PCMS | - | - | 3000 | 2.10 |
| PEUG20 | 20 | 20 | 31,000 | 2.22 |
| PEUG40 | 40 | 40 | 34,000 | 2.54 |
| PEUG60 | 60 | 60 | 37,000 | 2.69 |
| PEUG80 | 80 | 80 | 37,000 | 2.55 |
| PEUG100 | 150 | 100 | 38,000 | 2.48 |
| Polymer Designation | Contact Angle [o] 1 | Surface Energy [mJ/m2] 2 | LC Aligning Ability 3 | |||
|---|---|---|---|---|---|---|
| Water | Diiodomethane | Polar | Dispersion | Total | ||
| PCMS | 71.1 | 35.2 | 8.67 | 37.00 | 45.67 | X |
| PEUG20 | 85.2 | 23.8 | 1.97 | 44.70 | 46.66 | X |
| PEUG40 | 87.7 | 22.1 | 1.36 | 45.78 | 47.14 | X |
| PEUG60 | 88.8 | 22.0 | 1.08 | 46.10 | 47.18 | X |
| PEUG80 | 89.7 | 18.6 | 0.81 | 47.42 | 48.23 | O |
| PEUG100 | 90.1 | 18.5 | 0.75 | 47.53 | 48.28 | O |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, C.; Kim, T.; Kang, H. Renewable, Eugenol—Modified Polystyrene Layer for Liquid Crystal Orientation. Polymers 2018, 10, 201. https://doi.org/10.3390/polym10020201
Ju C, Kim T, Kang H. Renewable, Eugenol—Modified Polystyrene Layer for Liquid Crystal Orientation. Polymers. 2018; 10(2):201. https://doi.org/10.3390/polym10020201
Chicago/Turabian StyleJu, Changha, Taehyung Kim, and Hyo Kang. 2018. "Renewable, Eugenol—Modified Polystyrene Layer for Liquid Crystal Orientation" Polymers 10, no. 2: 201. https://doi.org/10.3390/polym10020201