Dynamic High-Pressure Microfluidization-Treated Pectin under Different Ethanol Concentrations
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Chemicals
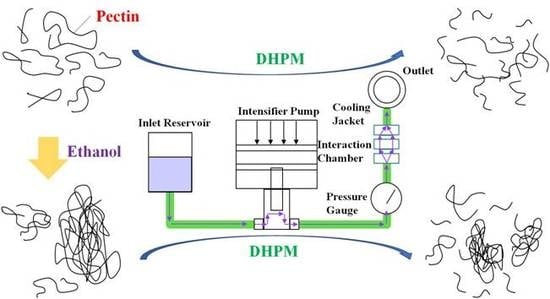
2.2. Preparation of Pectin Solution and DHPM Treatment
2.3. Characterization
2.3.1. Determination of Average Particle Size
2.3.2. Determination of Average Mw
2.3.3. Determination of Reducing-Sugar-Ends Content
2.3.4. Surface Morphology
2.3.5. Investigation of the Possible Reactions on Pectin Induced by DHPM
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Disaggregation and Degradation
3.1.1. Change of Average Particle Size
3.1.2. Change of Average Mw
3.1.3. Change of Reducing-Sugar-Ends Content
3.2. Change of Surface Topography
3.3. Characterization of Possible Reactions
3.4. Mechanism of Pectin Disaggregation and Degradation during DHPM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT-Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Singthong, J.; Ningsanond, S.; Cui, S.W.; Douglas Goff, H. Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocoll. 2005, 19, 793–801. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Garna, H.; Mabon, N.; Nott, K.; Wathelet, B.; Paquot, M. Kinetic of the hydrolysis of pectin galacturonic acid chains and quantification by ionic chromatography. Food Chem. 2006, 96, 477–484. [Google Scholar] [CrossRef]
- Albersheim, P. Instability of pectin in neutral solutions. Biochem. Biophys. Res. Commun. 1959, 1, 253–256. [Google Scholar] [CrossRef]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic degradation of citrus pectin and pectate during prolonged heating: Effects of pH, temperature, and degree of methyl esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Van Buggenhout, S.; Verlinden, B.E.; Christiaens, S.; Shpigelman, A.; Vicent, V.; Kermani, Z.J.; Nicolai, B.M.; Hendrickx, M.; Geeraerd, A. Pectin modifications and the role of pectin-degrading enzymes during postharvest softening of Jonagold apples. Food Chem. 2014, 158, 283–291. [Google Scholar] [CrossRef]
- Seshadri, R.; Weiss, J.; Hulbert, G.J.; Mount, J. Ultrasonic processing influences rheological and optical properties of high-methoxyl pectin dispersions. Food Hydrocoll. 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Van Deventer-Schriemer, W.; Pilnik, W. Studies on pectin degradation. Acta Aliment. 1987, 16, 143–153. [Google Scholar]
- Zhang, W.; Xie, F.; Lan, X.; Gong, S.; Wang, Z. Characteristics of pectin from black cherry tomato waste modified by dynamic high-pressure microfluidization. J. Food Eng. 2018, 216, 90–97. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Xie, M.; Liu, C.; Liu, W.; Wan, J. Characterization and high-pressure microfluidization-induced activation of polyphenoloxidase from Chinese pear (Pyrus pyrifolia Nakai). J. Agric. Food Chem. 2009, 57, 5376–5380. [Google Scholar] [CrossRef]
- Bouaouina, H.; Desrumaux, A.; Loisel, C.; Legrand, J. Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int. Dairy J. 2006, 16, 275–284. [Google Scholar] [CrossRef]
- Kasemwong, K.; Srinuanchai, W.; Itthisoponkul, T.; Sriroth, K. Effect of high-pressure microfluidization on the structure of cassava starch granule. Starch 2015, 63, 160–170. [Google Scholar] [CrossRef]
- Lagoueyte, N.; Paquin, P. Effects of microfluidization on the functional properties of xanthan gum. Food Hydrocoll. 1998, 12, 365–371. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Wang, H.; Kou, Y.; Wen, Q.H.; Fu, Z.F.; Chang, H.X. Response surface optimization and physicochemical properties of polysaccharides from Nelumbo nucifera leaves. Int. J. Biol. 2015, 74, 103–110. [Google Scholar] [CrossRef]
- Wan, J.; Liu, C.; Liu, W.; Tu, Z.; Wu, W.; Tan, H. Optimization of instant edible films based on dietary fiber processed with dynamic high pressure microfluidization for barrier properties and water solubility. LWT-Food Sci. Technol. 2015, 60, 603–608. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym 2018, 196. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, R.H.; Liu, W.; Liu, C.M.; Li, T.; Tu, Z.C.; Wan, J. Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocoll. 2012, 28, 121–129. [Google Scholar] [CrossRef]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Loey, A.M.V.; Hendrickx, M.E. The effect of high pressure homogenization on pectin: Importance of pectin source and pH. Food Hydrocoll. 2015, 43, 189–198. [Google Scholar] [CrossRef]
- Dentini, M.; Crescenzi, V.; Fidanza, M.; Coviello, T. The aggregation and conformational states in aqueous solution of a succinoglycan polysaccharide. Macromolecules 1989, 22, 954–959. [Google Scholar] [CrossRef]
- Exarhopoulos, S.; Raphaelides, S.N.; Kontominas, M.G. Conformational studies and molecular characterization of the polysaccharide kefiran. Food Hydrocoll. 2018, 77, 347–356. [Google Scholar] [CrossRef]
- Antoniou, E.; Alexandridis, P. Polymer conformation in mixed aqueous-polar organic solvents. Eur. Polym. J. 2010, 46, 324–335. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- National Academy of Science. Food Chemical Codex; National Academy of Science: Washington, DC, USA, 1981; pp. 283–286. [Google Scholar]
- Liu, C.M.; Zhong, J.Z.; Liu, W.; Tu, Z.C.; Wan, J.; Cai, X.F.; Song, X.Y. Relationship between functional properties and aggregation changes of whey protein induced by high pressure microfluidization. J. Food Sci. 2011, 76, E341–E347. [Google Scholar] [CrossRef] [PubMed]
- Floury, J.; Desrumaux, A.; Axelos, M.A.; Legrand, J. Degradation of methylcellulose during ultra-high pressure homogenisation. Food Hydrocoll. 2002, 16, 47–53. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Munarin, F.; Bozzini, S.; Visai, L.; Tanzi, M.C.; Petrini, P. Sterilization treatments on polysaccharides: Effects and side effects on pectin. Food Hydrocoll. 2013, 31, 74–84. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Zhao, D.; Lau, E.; Padilla-Zakour, O.I.; Moraru, C.I. Role of pectin and haze particles in membrane fouling during cold microfiltration of apple cider. J. Food Eng. 2017, 200, 47–58. [Google Scholar] [CrossRef]
- Croak, S.; Corredig, M. The role of pectin in orange juice stabilization: Effect of pectin methylesterase and pectinase activity on the size of cloud particles. Food Hydrocoll. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Sagis, L.M.C.; de Vries, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench). Food Hydrocoll. 2010, 24, 35–41. [Google Scholar] [CrossRef]
- Rimada, P.S.; Abraham, A.G. Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Le Lait 2003, 83, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Visser, J. Van der Waals and other cohesive forces affecting powder fluidization. Powder Technol. 1989, 58, 1–10. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Sakata, M.; McKenna, C.; Aoki, H.; Phillips, G.O. Molecular associations in acacia gums. Struct. Chem. 2009, 20, 325. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Xie, M.Y. High pressure homogenization increases antioxidant capacity and short-chain fatty acid yield of polysaccharide from seeds of Plantago asiatica L. Food Chem. 2013, 138, 2338–2345. [Google Scholar] [CrossRef]
- Liang, R.H.; Chen, J.; Liu, W.; Liu, C.M.; Yu, W.; Yuan, M.; Zhou, X.Q. Extraction, characterization and spontaneous gel-forming property of pectin from creeping fig (Ficus pumila Linn) seeds. Carbohydr. Polym. 2012, 87, 76–83. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–4626. [Google Scholar] [CrossRef]
- Jiang, C.M.; Li, C.P.; Lee, B.H.; Chang, J.C.; Chang, H.M. Changes in physico-chemical properties of pectin from jelly fig (Ficus awkeotsang Makino) seeds during extraction and gelling. Food. Res. Int. 2002, 35, 31–35. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Bajer, K.; Gałka, P.; Kotnowska, B. Photodegradation studies of novel biodegradable blends based on poly (ethylene oxide) and pectin. Polym. Degrad. Stabil. 2007, 92, 2058–2069. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Yi, J.; Peng, J.; Ning, C.; Wellala, C.K.D.; Zhang, B. Effects of high pressure homogenization on pectin structural characteristics and carotenoid bioaccessibility of carrot juice. Carbohydr. Polym. 2019, 203, 176–184. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-M.; Liang, L.; Shuai, X.-X.; Liang, R.-H.; Chen, J. Dynamic High-Pressure Microfluidization-Treated Pectin under Different Ethanol Concentrations. Polymers 2018, 10, 1410. https://doi.org/10.3390/polym10121410
Liu C-M, Liang L, Shuai X-X, Liang R-H, Chen J. Dynamic High-Pressure Microfluidization-Treated Pectin under Different Ethanol Concentrations. Polymers. 2018; 10(12):1410. https://doi.org/10.3390/polym10121410
Chicago/Turabian StyleLiu, Cheng-Mei, Lu Liang, Xi-Xiang Shuai, Rui-Hong Liang, and Jun Chen. 2018. "Dynamic High-Pressure Microfluidization-Treated Pectin under Different Ethanol Concentrations" Polymers 10, no. 12: 1410. https://doi.org/10.3390/polym10121410