Naturally-Derived Amphiphilic Polystyrenes Prepared by Aqueous Controlled/Living Cationic Polymerization and Copolymerization of Vinylguaiacol with R–OH/BF3·OEt2
Abstract
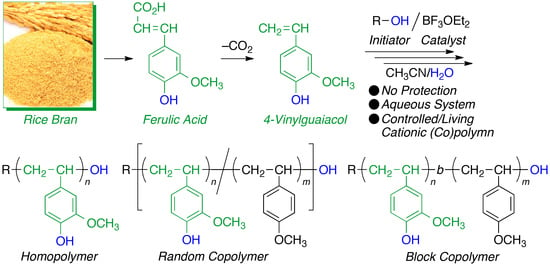
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aqueous Cationic Polymerization of 4-vinylguaiacol (4VG)
2.3. Block Copolymerization of 4VG and pMOS with the R–OH/BF3·OEt2 System
2.4. Measurements
3. Results and Discussion
3.1. Poly(vinylguaiacol) via Aqueous Cationic Polymerization
3.2. Statistical and Block Copolymers of Vinylguaiacol with p-Methoxystyrene
3.3. Properties of the Naturally-Derived Phenolic Polymers from Vinylguaiacol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, A.H.E.; Matyjaszewski, K. (Eds.) Controlled and Living Polymerizations: From Mechanisms to Applications; Wiley-VCH: Weinheim, Germany, 2010; ISBN 978-3-5273-2492-7. [Google Scholar]
- Sawamoto, M. Modern cationic vinyl polymerization. Prog. Polym. Sci. 1991, 16, 111–172. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Iván, B. Designed Polymers by Carbocationic Macromolecular Engineering: Theory and Practice; Hanser: Munich, Germany, 1992; ISBN 0195209214. [Google Scholar]
- Matyjaszewski, K. (Ed.) Cationic Polymerizations: Mechanisms, Synthesis, and Applications; Marcel Dekker: New York, NY, USA, 1996; ISBN 082479463X. [Google Scholar]
- Puskas, J.E.; Kaszas, G. Living carbocationic polymerization of resonance-stabilized monomers. Prog. Polym. Sci. 2000, 25, 403–452. [Google Scholar] [CrossRef]
- Goethals, E.J.; Du Prez, F. Carbocationic polymerizations. Prog. Polym. Sci. 2007, 32, 220–246. [Google Scholar] [CrossRef]
- Aoshima, S.; Kanaoka, S. A Renaissance in Living Cationic Polymerization. Chem. Rev. 2009, 109, 5245–5287. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Kamigaito, M.; Sawamoto, M. Direct Living Cationic Polymerization of p-Hydroxystyrene with Boron Trifluoride Etherate in the Presence of Water. Macromolecules 2000, 33, 5405–5410. [Google Scholar] [CrossRef]
- Satoh, K.; Kamigaito, M.; Sawamoto, M. Direct Synthesis of Amphiphilic Random and Block Copolymers of p-Hydroxystyrene and p-Methoxystyrene via Living Cationic Polymerization with BF3OEt2/ROH Systems. Macromolecules 2000, 33, 5830–5835. [Google Scholar] [CrossRef]
- Satoh, K.; Nakashima, J.; Kamigaito, M.; Sawamoto, M. Novel BF3OEt2/R−OH Initiating System for Controlled Cationic Polymerization of Styrene in the Presence of Water. Macromolecules 2001, 34, 396–401. [Google Scholar] [CrossRef]
- Kamigaito, M.; Nakashima, J.; Satoh, K.; Sawamoto, M. Controlled Cationic Polymerization of p-(Chloromethyl)styrene: BF3-Catalyzed Selective Activation of a C−O Terminal from Alcohol. Macromolecules 2003, 36, 3540–3544. [Google Scholar] [CrossRef]
- Satoh, K. Cationic Polymerization in Aqueous Media with Water-Tolerant Lewis Acids. Kobunshi Ronbunshu 2005, 62, 335–351. [Google Scholar] [CrossRef] [Green Version]
- Kostjuk, S.V.; Radchenko, A.V.; Ganachaud, F. Controlled/Living Cationic Polymerization of p-Methoxystyrene in Solution and Aqueous Dispersion Using Tris(pentafluorophenyl)borane as a Lewis Acid: Acetonitrile Does the Job. Macromolecules 2007, 40, 482–490. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Ganachaud, F. Cationic Polymerization of Vinyl Monomers in Aqueous Media: From Monofunctional Oligomers to Long-Lived Polymer Chains. Acc. Chem. Res. 2010, 43, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Kimura, Y. Molecular, Stractural, and Material Design of Bio-Based Polymers. Polym. J. 2009, 41, 797–807. [Google Scholar] [CrossRef]
- Biermann, U.; Bornsheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Themosetting Epoxy; Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation. Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Miller, S.A. Sustainable Polymers: Opportunities for the Next Decade. ACS Macro Lett. 2013, 2, 550–554. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Reno, K.H.; Wool, R.P.; Epps, T.H., III. Biobased building blocks for the rational design of renewable block polymers. Soft Matter. 2014, 10, 7405–7424. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Dannecker, P.-K.; von Czapiewski, M.; Over, L.C.; Söyer, Z.; Meier, M.A.R. Renewability is not Enough: Recent Advances in the Sustainable Synthesis of Biomass-Derived Monomers and Polymers. Chem. Eur. J. 2016, 22, 11510–11521. [Google Scholar] [CrossRef] [PubMed]
- Thomsett, M.R.; Storr, T.E.; Monaghan, O.R.; Stockman, R.A.; Howdle, S.M. Progress in the sustainable polymers from terpenes and terpenoids. Green Mater. 2016, 4, 115–134. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, D.K.; Hillmyer, M.A. There is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Satoh, K.; Kamigaito, M. New Polymerization Methods for Biobased Polymers. In Bio-Based Polymers; Kimura, Y., Ed.; CMC: Tokyo, Japan, 2013; pp. 95–111. ISBN 978-4-7813-0271-3. [Google Scholar]
- Kamigaito, M.; Satoh, K. Bio-based Hydrocarbon Polymers. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Heidelberg, Germany, 2015; Volume 1, pp. 109–118. ISBN 978-3-642-29649-9. [Google Scholar]
- Kamigaito, M.; Satoh, K. Sustainable Vinyl Polymers via Controlled Polymerization of Terpenes. In Sustainable Polymers from Biomass; Tang, C., Ryu, C.Y., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 55–90. ISBN 978-3-5273-4016-3. [Google Scholar]
- Satoh, K. Controlled/living polymerization of renewable vinyl monomers into bio-based polymers. Polym. J. 2015, 47, 527–536. [Google Scholar] [CrossRef]
- Satoh, K.; Sugiyama, H.; Kamigaito, M. Biomass-derived heat-resistant hydrogenated alicyclic hydrocarbon polymers: Poly(terpenes) and their derivatives. Green Chem. 2006, 8, 878–882. [Google Scholar] [CrossRef]
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for poly(β-pinene). Optoelectronics material and catalytic hydrogenation for high-molecular-weight hydrogenated: Living cationic polymerization of β-pinene. Polym. Chem. 2014, 5, 3222–3230. [Google Scholar] [CrossRef]
- Miyaji, H.; Satoh, K.; Kamigaito, M. Bio-Based Polyketones by Selective Ring-Opening Radical Polymerization of α-Pinene-Derived Pinocarvone. Angew. Chem. Int. Ed. 2016, 55, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Matsuda, M.; Nagai, K.; Kamigaito, M. AAB-Sequence Living Radical Chain Copolymerization of Naturally-Occurring Limonene with Maleimide: An End-to-End Sequence- Regulated Copolymer. J. Am. Chem. Soc. 2010, 132, 10003–10005. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Satoh, K.; Kamigaito, M. Periodically Functionalized and Grafted Copolymers via 1:2-Sequence-Regulated Radical Copolymerization of Naturally Occurring Functional Limonene and Maleimide Derivatives. Macromolecules 2013, 46, 5473–5482. [Google Scholar] [CrossRef]
- Matsuda, M.; Satoh, K.; Kamigaito, M. Controlled Radical Copolymerization of Naturally-Occurring Terpenes with Acrylic Monomers in Fluorinated Alcohol. KGK Kaut. Gummi Kunstst. 2013, 66, 51–56. [Google Scholar]
- Matsuda, M.; Satoh, K.; Kamigaito, M. 1:2-sequence-regulated radical copolymerization of naturally occurring terpenes with maleimide derivatives in fluorinated alcohol. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 1774–1785. [Google Scholar] [CrossRef]
- Ojika, M.; Satoh, K.; Kamigaito, M. BAB-random-C Monomer Sequence via Radical Terpolymerization of Limonene (A), Maleimide (B), and Methacrylate (C): Terpene Polymers with Randomly Distributed Periodic Sequences. Angew. Chem. Int. Ed. 2017, 56, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Saitoh, S.; Kamigaito, M. A Linear Lignin Analogue: Phenolic Alternating Copolymers from Naturally Occurring β-Methylstyrene via Aqueous-Controlled Cationic Copolymerization. J. Am. Chem. Soc. 2007, 129, 9586–9587. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, Y.; Satoh, K.; Kamigaito, M. Renewable β-methylstyrenes for bio-based heat-resistant styrenic copolymers: Radical copolymerization enhanced by fluoroalcohol and controlled/living copolymerization by RAFT. Polym. Chem. 2014, 5, 3182–3189. [Google Scholar] [CrossRef]
- Satoh, K.; Lee, D.-H.; Nagai, K.; Kamigaito, M. Precision Synthesis of Bio-Based Acrylic Thermoplastic Elastomer by RAFT Polymerization of Itaconic Acid Derivatives. Macromol. Rapid Commun. 2014, 35, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Bio-Based Functional Styrene Monomers Derived from Naturally Occurring Ferulic Acid for Poly(vinylcatechol) and Poly(vinylguaicol) via Controlled Radical Polymerization. Macromolecules 2017, 50, 4206–4216. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Scalable Synthesis of Bio-Based Functional Styrene: Protected Vinyl Catechol from Caffeic Acid and Controlled Radical and Anionic Polymerizations Thereof. ACS. Sustain. Chem. Eng. 2018, 6, 13681–13686. [Google Scholar] [CrossRef]
- Terao, Y.; Satoh, K.; Kamigaito, M. Controlled Radical Copolymerization of Cinnamic Derivatives as Renewable Vinyl Monomers with Both Acrylic and Styrenic Substituents: Reactivity, Regioselectivity, Properties, and Functions. Biomacromolecules. in press. [CrossRef] [PubMed]
- Salvin, S.; Bourke, M.; Byrne, T. (Eds.) The New Crop Industries Handbook; Rural Industries Research and Development Corporation: Canberra, Autralia, 2004; ISBN 1-74151-610-2. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. (Eds.) Handbook of Essential Oils, 2nd ed.; CRC Press: Boca Barton, FL, USA, 2016; ISBN 978-1-4665-9046-5. [Google Scholar]
- Caisamigila, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. Adv. Polym. Sci. 2009, 232, 1–63. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, L.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sachan, A.; Vidyarthi, A.S.; Sachan, S.G. Transformation of ferulic acid to 4-vinyl guaiacol as a major metabolite: A microbial approach. Rev. Environ. Sci. Bio/Technol. 2014, 13, 377–385. [Google Scholar] [CrossRef]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing: Science and Practice; CRC Press: Boca Raton, FL, USA, 2004; pp. 610–632. ISBN 978-1-8557-3490-6. [Google Scholar]
- Mukai, N.; Masaki, K.; Fujii, T.; Iefuji, H. Single nucleotide polymorphisms of PAD1 and FDC1 show a positive relationship with ferulic acid decarboxylation ability among industrial yeasts used in alcoholic beverage production. Biosci. Bioeng. 2014, 118, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.A.; Jones, W.M. A Study of pH Dependence in the Decarboxylation of p-Hydroxycinnamic Acid. J. Am. Chem. Soc. 1960, 82, 1907–1911. [Google Scholar] [CrossRef]
- Nomura, E.; Hosoda, A.; Mori, H.; Taniguchi, H. Rapid base-catalyzed decarboxylation and amide-forming reaction of substituted cinnamic acids via microwave heating. Green Chem. 2005, 7, 863–866. [Google Scholar] [CrossRef]







| Polymer b | H2O | NaOH aq | CH3OH | Acetone | THF | EtOAc | CHCl3 | Toluene |
|---|---|---|---|---|---|---|---|---|
| Poly(4VG) | − | ++ | ++ | ++ | ++ | ++ | − | − |
| Poly(pMOS) | − | − | − | ++ | ++ | ++ | ++ | ++ |
| Poly(4VG-stat-pMOS) | − | + | − | ++ | ++ | ++ | ++ | − |
| Poly(4VG-block-pMOS) | − | − | − | ++ | ++ | ++ | ++ | − |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshima, H.; Satoh, K.; Kamigaito, M. Naturally-Derived Amphiphilic Polystyrenes Prepared by Aqueous Controlled/Living Cationic Polymerization and Copolymerization of Vinylguaiacol with R–OH/BF3·OEt2. Polymers 2018, 10, 1404. https://doi.org/10.3390/polym10121404
Takeshima H, Satoh K, Kamigaito M. Naturally-Derived Amphiphilic Polystyrenes Prepared by Aqueous Controlled/Living Cationic Polymerization and Copolymerization of Vinylguaiacol with R–OH/BF3·OEt2. Polymers. 2018; 10(12):1404. https://doi.org/10.3390/polym10121404
Chicago/Turabian StyleTakeshima, Hisaaki, Kotaro Satoh, and Masami Kamigaito. 2018. "Naturally-Derived Amphiphilic Polystyrenes Prepared by Aqueous Controlled/Living Cationic Polymerization and Copolymerization of Vinylguaiacol with R–OH/BF3·OEt2" Polymers 10, no. 12: 1404. https://doi.org/10.3390/polym10121404