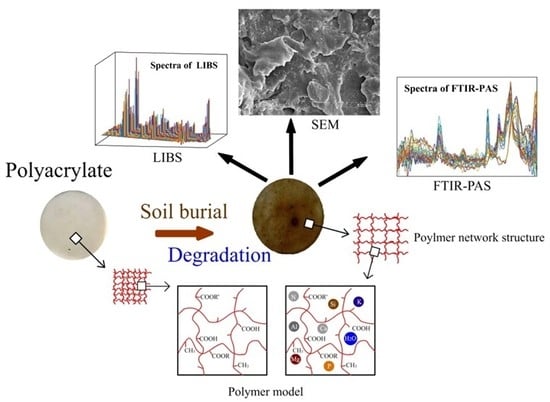
Degradation of Polyacrylate in the Outdoor Agricultural Soil Measured by FTIR-PAS and LIBS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Outdoor Soil Burial Test
2.3. SEM
2.4. FTIR-PAS
2.5. LIBS
2.6. PCA
3. Results and Discussion
3.1. Weight Loss and Morphological Properties
3.2. FTIR-PAS Spectra
3.3. LIBS Spectra
3.4. PCA of FTIR-PAS and LIBS
3.5. The Degradation Process of PA in Soil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robeson, L.M. Polymer Blends: A Comprehensive Review, 1st ed.; Carl Hanser Verlag: Munich, Germany, 2007; pp. 109–210. ISBN 978-3-446-43650-3. [Google Scholar]
- Shen, Y.; Zhao, C.; Zhou, J.; Du, C. Application of waterborne acrylic emulsions in coated controlled release fertilizer using reacted layer technology. Chin. J. Chem. Eng. 2015, 23, 309–314. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.; Zhou, J. Aqueous polyacrylate/poly(silicone-co-acrylate) emulsion coated fertilizers for slow nutrient-release application. J. Appl. Polym. Sci. 2014, 131, 40369–40376. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.; Zhou, J.; Ma, F. Application of nano feiii-tannic acid complexes in modifying aqueous acrylic latex for controlled-release coated urea. J. Agric. Food Chem. 2017, 65, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shen, Y.; Du, C.; Zhou, J.; Qin, Y.; Wu, Y. Economic and soil environmental benefits of using controlled-release bulk blending urea in the north china plain. Land Degrad. Dev. 2017, 28, 2370–2379. [Google Scholar] [CrossRef]
- Geesing, D.; Schmidhalter, U. Influence of sodium polyacrylate on the water-holding capacity of three different soils and effects on growth of wheat. Soil Use Manag. 2004, 20, 207–209. [Google Scholar] [CrossRef]
- Guiwei, Q.; de Varennes, A.; Cunha-Queda, C. Remediation of a mine soil with insoluble polyacrylate polymers enhances soil quality and plant growth. Soil Use Manag. 2008, 24, 350–356. [Google Scholar] [CrossRef]
- Zhou, Z.; Du, C.; Li, T.; Shen, Y.; Zeng, Y.; Du, J.; Zhou, J. Biodegradation of a biochar-modified waterborne polyacrylate membrane coating for controlled-release fertilizer and its effects on soil bacterial community profiles. Environ. Sci. Pollut. Res. Int. 2015, 22, 8672–8682. [Google Scholar] [CrossRef] [PubMed]
- Wilske, B.; Bai, M.; Lindenstruth, B.; Bach, M.; Rezaie, Z.; Frede, H.-G.; Breuer, L. Biodegradability of a polyacrylate superabsorbent in agricultural soil. Environ. Sci. Pollut. Res. Int. 2014, 21, 9453–9460. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.D.; Cameron, M.D.; Haselbach, J.; Aust, S.D. Biodegradation of superabsorbent polymers in soil. Environ. Sci. Pollut. Res. Int. 2000, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zeng, Y.; Du, C.; Shen, Y.; Ma, H.; Xu, S.; Zhou, J. Soil variability description using fourier transform mid-infrared photoacoustic spectroscopy coupling with rgb method. CATENA 2017, 152, 190–197. [Google Scholar] [CrossRef]
- Lu, Y.; Du, C.; Yu, C.; Zhou, J. Fast and nondestructive determination of protein content in rapeseeds (brassica napus l.) using fourier transform infrared photoacoustic spectroscopy (ftir-pas). J. Sci. Food Agric. 2014, 94, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Du, C.; Ma, F.; Shen, Y.; Wu, K.; Zhou, J. Characterization of nano feiii-tannic acid modified polyacrylate in controlled-release coated urea by fourier transform infrared photoacoustic spectroscopy and laser-induced breakdown spectroscopy. Polym. Test. 2017, 64, 101–108. [Google Scholar] [CrossRef]
- Du, C.; Zhou, J.; Liu, J. Identification of chinese medicinal fungus cordyceps sinensis by depth-profiling mid-infrared photoacoustic spectroscopy. Spectrochim. Acta Part A 2017, 173, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, C.; Zeng, Y.; Ma, F.; Shen, Y.; Xing, Z.; Zhou, J. Two-dimensional visualization of nitrogen distribution in leaves of chinese cabbage (brassica rapa subsp. Chinensis) by the fourier transform infrared photoacoustic spectroscopy technique. J. Agric. Food Chem. 2016, 64, 7696–7701. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhou, J. Evaluation of soil fertility using infrared spectroscopy: A review. Environ. Chem. Lett. 2009, 7, 97–113. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Speciation and surface structure of inorganic arsenic in solid phases: A review. Environ. Int. 2008, 34, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Du, C.; Zhou, J.; Ma, F. The facile modification of polyacrylate emulsion via hexadecane to enhance controlled-release profiles of coated urea. Sci. Rep. 2018, 8, 12279. [Google Scholar] [CrossRef] [PubMed]
- Shidan, B. Agrochemical Analysis Methods in Soil Science; Agricultural Publisher: Beijing, China, 2010; ISBN 9787109066441. [Google Scholar]
- Du, C.; Zhou, J. Application of infrared photoacoustic spectroscopy in soil analysis. Appl. Spectrosc. Rev. 2011, 46, 405–422. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Vukovic-Kwiatkowska, I. Preparation and characterization of interpenetrating networks based on polyacrylates and poly(lactic acid). Express Polym. Lett. 2012, 6, 78–94. [Google Scholar] [CrossRef] [Green Version]






| Assignment | Wavenumber (cm−1) | Thermal Diffusion Length (μm) |
|---|---|---|
| O–H stretching (–OH) | 3320 | 5.48 |
| C–H stretching (–CH3) C–H stretching (–CH2 ) | 2950 2860 | 5.81 5.90 |
| C=O stretching (–COOR) | 1730 | 7.58 |
| C–H deformation (–CH3, –CH2–) | 1450 | 8.28 |
| C–O–C stretching (–COOR) | 1160 | 9.26 |
| Si–O–Si stretching (–SiO–) | 1030 | 9.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Du, C.; Ma, F.; Shen, Y.; Wu, K.; Zhou, J. Degradation of Polyacrylate in the Outdoor Agricultural Soil Measured by FTIR-PAS and LIBS. Polymers 2018, 10, 1296. https://doi.org/10.3390/polym10121296
Liang D, Du C, Ma F, Shen Y, Wu K, Zhou J. Degradation of Polyacrylate in the Outdoor Agricultural Soil Measured by FTIR-PAS and LIBS. Polymers. 2018; 10(12):1296. https://doi.org/10.3390/polym10121296
Chicago/Turabian StyleLiang, Dong, Changwen Du, Fei Ma, Yazhen Shen, Ke Wu, and Jianmin Zhou. 2018. "Degradation of Polyacrylate in the Outdoor Agricultural Soil Measured by FTIR-PAS and LIBS" Polymers 10, no. 12: 1296. https://doi.org/10.3390/polym10121296