Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue
Abstract
:1. Introduction
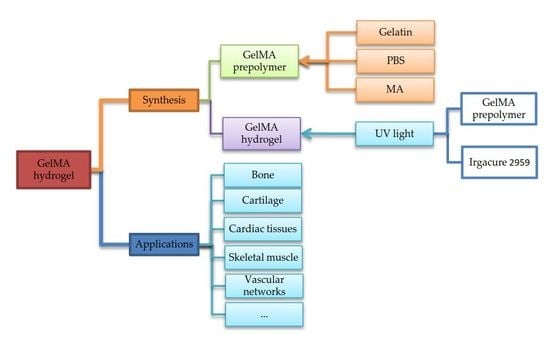
2. Synthesis and Characterization of GelMA Hydrogels
2.1. Synthesis
2.2. Characterization and Property Control
2.2.1. Degree of Substitution
2.2.2. Chemical Groups
2.2.3. Morphology
2.2.4. Stability
2.2.5. Wettability
2.2.6. Mechanical Properties
2.2.7. Swelling Property
2.2.8. Degradability
2.2.9. Viscoelasticity
3. Applications of GelMA Hydrogels
3.1. Cell Culture
3.2. Applications in Load-Bearing Tissue
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.J.; Yuan, Y.F.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Deligkaris, K.; Tadele, T.S.; Olthuis, W.; van den Berg, A. Hydrogel-based devices for biomedical applications. Sens. Actuator B 2010, 147, 765–774. [Google Scholar] [CrossRef]
- Miri, A.K.; Hosseinabadi, H.G.; Cecen, B.; Hassan, S.; Zhang, Y.S. Permeability mapping of gelatin methacryloyl hydrogels. Acta Biomater. 2018, 77, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated hydrogels for tissue engineering and regenerative medicine. Bioconjug. Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Van den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, P.E.; Huff, T.J.; Zuniga, J.M. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J. Tissue Eng. 2018, 9, 2041731418769863. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Santiago, G.T.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, L.; Peng, H.T.; Pang, N.S. Development of a Novel Biomaterial: Part II. Evaluation of a Photo Cross-Linking Method; DRDC: Toronto, ON, Canada, 2005. [Google Scholar]
- Lee, B.H.; Shirahama, H.; Cho, N.J.; Tan, L.P. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J.A. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed]
- Zongjie, W.; Zhenlin, T.; Xian, J.; Holzman, J.F.; Menard, F.; Keekyoung, K. Visible light-based stereolithography bioprinting of cell-adhesive gelatin hydrogels. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Seogwipo, South Korea, 11–15 July 2017; pp. 1599–1602. [Google Scholar]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible light photoinitiation of cell-adhesive gelatin Methacryloyl hydrogels for stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef] [PubMed]
- Erkoc, P.; Seker, F.; Bagci-Onder, T.; Kizilel, S. Gelatin methacryloyl hydrogels in the absence of a crosslinker as 3D glioblastoma multiforme (GBM)-mimetic microenvironment. Macromol. Biosci. 2018, 18, 1700369. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Lujan, T.J.; Hsu, C.W.; Bottlang, M.; West, J.L.; Johnstone, B. Visible Light Photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cells Mater. 2011, 22, 43–55. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Tan, J.; Sun, H.; Yu, M.; Sumerlin, B.S.; Zhang, L. Photo-PISA: Shedding light on polymerization-induced self-assembly. ACS Macro Lett. 2015, 4, 1249–1253. [Google Scholar] [CrossRef]
- Yang, L.; Tang, H.; Sun, H. Progress in photo-responsive polypeptide derived nano-assemblies. Micromachines 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter. 2011, 7, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef]
- Rahali, K.; Ben Messaoud, G.; Kahn, C.J.F.; Sanchez-Gonzalez, L.; Kaci, M.; Cleymand, F.; Fleutot, S.; Linder, M.; Desobry, S.; Arab-Tehrany, E. Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2017, 18, 2675. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Cnudde, V.; Dubruel, P.; Masschaele, B.; Cosijns, A.; De Paepe, I.; Jacobs, P.J.S.; Van Hoorebeke, L.; Remon, J.P.; Schacht, E. Porous gelatin hydrogels: 1. Cryogenic formation and structure analysis. Biomacromolecules 2007, 8, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Effect of cryogenic treatment on the rheological properties of gelatin hydrogels. J. Bioact. Compat. Polym. 2010, 25, 498–512. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Lin, R.-Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. Part A 2018, 106, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.J.; Hasselbrink, E.F., Jr. Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis 2004, 25, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Ogawa, R.; Kakugo, A.; Osada, Y.; Gong, J.P. Dynamic cell behavior on synthetic hydrogels with different charge densities. Soft Matter. 2009, 5, 1804–1811. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Lehocky, M.; Vitkova, E.; Saha, P. Significant characteristics of medical-grade polymer sheets and their efficiency in protecting hydrogel wound dressings: A soft polymeric biomaterial. Int. J. Polym. Mater. 2012, 61, 72–88. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Bilek, F.; Vesel, A.; Junkar, I.; Saha, P.; Popelka, A. Polysaccharides coatings on medical-grade PVC: A probe into surface characteristics and the extent of bacterial adhesion. Molecules 2010, 15, 1007–1027. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Tanaka, J. XPS study for the microstructure development of hydroxyapatite–collagen nanocomposites cross-linked using glutaraldehyde. Biomaterials 2002, 23, 3879–3885. [Google Scholar] [CrossRef]
- Tan, G.; Zhou, L.; Ning, C.; Tan, Y.; Ni, G.; Liao, J.; Yu, P.; Chen, X. Biomimetically-mineralized composite coatings on titanium functionalized with gelatin methacrylate hydrogels. Appl. Surf. Sci. 2013, 279, 293–299. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Du, Y.; Wang, L.; Kaji, H.; Bae, H.; Khademhosseini, A. Patterned differentiation of individual embryoid bodies in spatially organized 3D hybrid microgels. Adv. Mater. 2010, 22, 5276–5281. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Azcón, J.; Ahadian, S.; Obregón, R.; Camci-Unal, G.; Ostrovidov, S.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; et al. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip 2012, 12, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Chung, P.H.; Soman, P.; Chen, P.; Lotz, M.K.; Chen, S.; D’Lima, D.D. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013, 9, 7218–7226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.; Oh, J. Swelling characterization of photo-cross-linked gelatin methacrylate spherical microgels for bioencapsulation. E-Polymers 2014, 14, 161. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Dubruel, P.; Möller, L.; Dräger, G.; Chichkov, B. Laser fabrication of three-Dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.-C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-Cross-Linkable Methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl. Mater. Interfaces 2015, 7, 10386–10394. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Manoharan, V.; Krafft, D.; Assmann, A.; Uquillas, J.A.; Shin, S.R.; Hasan, A.; Hussain, M.A.; Memic, A.; Gaharwar, A.K.; et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 2016, 4, 3544–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, T.; Xavier, J.R.; Cross, L.; Jaiswal, M.K.; Mondragon, E.; Kaunas, R.; Gaharwar, A.K. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J. Biomed. Mater. Res. Part A 2016, 104, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; He, X.; Yang, Y.; Wei, D.; Sun, J.; Zhong, M.; Xie, R.; Fan, H.; Zhang, X. Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction. Acta Biomater. 2016, 38, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Guo, J.; Guan, Y.; Zhang, H.; Li, X.; Chen, X.; Zhou, D.; Huang, Y. Development of organic/inorganic compatible and sustainably bioactive composites for effective bone regeneration. Biomacromolecules 2018, 19, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Moises Alvarez, M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xiao, W.; Sun, J.; Zhong, M.; Guo, L.; Fan, H.; Zhang, X. A biocompatible hydrogel with improved stiffness and hydrophilicity for modular tissue engineering assembly. J. Mater. Chem. B 2015, 3, 2753–2763. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a photo-crosslinking, biodegradable GelMA/PEGDA hydrogel for guided bone regeneration materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, F.; Zhang, W.; Mo, Y.; Zeng, L.; Li, X.; Chen, X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 89, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xie, J.; Zhong, L.; Li, J.; Rong, D.; Li, X.; Ouyang, J. Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1070–1080. [Google Scholar] [CrossRef]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Cui, W.; Weitz, D. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Visser, J.; Gawlitta, D.; Benders, K.E.M.; Toma, S.M.H.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Akkineni, A.; Gelinsky, M.; Woodruff, M.; Klein, T. A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering. Materials 2016, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, J.; Ye, P.; Gao, G.; Hubbell, K.; Cui, X. Coculture of mesenchymal stem cells and endothelial cells enhances host tissue integration and epidermis maturation through AKT activation in gelatin methacryloyl hydrogel-based skin model. Acta Biomater. 2017, 59, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, M.J.; Ki, C.S.; Kim, H.J.; Park, Y.H. Fabrication of bi-layer scaffold of keratin nanofiber and gelatin-methacrylate hydrogel: Implications for skin graft. Int. J. Biol. Macromol. 2017, 105, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xing, T.L.; Zhang, H.B.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018, 13, 035008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z.; et al. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D.; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Raman, R.; Cvetkovic, C.; Bashir, R. Enabling microscale and nanoscale approaches for bioengineered cardiac tissue. ACS Nano 2013, 7, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yuksekkaya, M.; Wan, K.-T.; et al. Reduced graphene oxide-gelma hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Yamada, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, P.; Kaczmarek, J.A.; Head, S.E.; Ellis, M.E.; Seeto, W.J.; Kim, J.; Bhattacharya, S.; Suppiramaniam, V.; Lipke, E.A. Direct production of human cardiac tissues by pluripotent stem cell encapsulation in gelatin Methacryloyl. ACS Biomater. Sci. Eng. 2017, 3, 1499–1509. [Google Scholar] [CrossRef]
- Alemdar, N.; Leijten, J.; Camci-Unal, G.; Hjortnaes, J.; Ribas, J.; Paul, A.; Mostafalu, P.; Gaharwar, A.K.; Qiu, Y.; Sonkusale, S.; et al. Oxygen-generating photo-cross-linkable hydrogels support cardiac progenitor cell survival by reducing hypoxia-induced necrosis. ACS Biomater. Sci. Eng. 2017, 3, 1964–1971. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boere, K.W.M.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; van Steenbergen, M.J.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.-H. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-E.; Kang, B.J.; Kim, S.-H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Han, L.; Xu, J.; Lu, X.; Gan, D.; Wang, Z.; Wang, K.; Zhang, H.; Yuan, H.; Weng, J. Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J. Mater. Chem. B 2017, 5, 731–741. [Google Scholar] [CrossRef]
- Brown, G.C.J.; Lim, K.S.; Farrugia, B.L.; Hooper, G.J.; Woodfield, T.B.F. Covalent incorporation of heparin improves chondrogenesis in photocurable gelatin-methacryloyl hydrogels. Macromol. Biosci. 2017, 17, 1700158. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Dautzenberg, N.M.M.; Levato, R.; van Rijen, M.H.P.; Dhert, W.J.A.; Malda, J.; Gawlitta, D. Ex vivo model unravelling cell distribution effect in hydrogels for cartilage repair. Altex Altern. Anim. Exp. 2018, 35, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Cui, H.T.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chen, Y.-C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, P.; Yuan, A.; Nam, K.-H.; Jiao, A.; Kim, D.-H. Fabrication of poly(ethylene glycol): Gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication 2014, 6, 024112. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Shridhar, S.V.; Gerwitz, M.; Soman, P. An in vitro vascular chip using 3D printing-enabled hydrogel casting. Biofabrication 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.S.; Pi, Q.; van Genderen, A.M. Microfluidic bioprinting for engineering vascularized tissues and organoids. J. Vis. Exp. 2017, 126, e55957. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; He, W.; Franca, C.M.; Athirasala, A.; Bertassoni, L.E. Engineering microvascular networks in LED light-cured cell-laden hydrogels. ACS Biomater. Sci. Eng. 2018, 4, 2563–2570. [Google Scholar] [CrossRef]
- Zhao, Q.; Cui, H.; Wang, J.; Chen, H.; Wang, Y.; Zhang, L.; Du, X.; Wang, M. Regulation effects of biomimetic hybrid scaffolds on vascular endothelium remodeling. ACS Appl. Mater. Interfaces 2018, 10, 23583–23594. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Ramón-Azcón, J.; Ostrovidov, S.; Camci-Unal, G.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Interdigitated array of Pt electrodes for electrical stimulation and engineering of aligned muscle tissue. Lab Chip 2012, 12, 3491–3503. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Ramon-Azcon, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.; et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci. Rep. 2014, 4, 4271. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Fornetti, E.; Barbetta, A.; Trombetta, M.; Cannata, S.M.; Gargioli, C.; Rainer, A. Engineering muscle networks in 3D gelatin methacryloyl hydrogels: Influence of mechanical stiffness and geometrical confinement. Front. Bioeng. Biotechnol. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Ostrovidov, S.; Salehi, S.; Kim, S.B.; Bae, H.; Khademhosseini, A. Enhanced skeletal muscle formation on microfluidic spun gelatin methacryloyl (GelMA) fibers using surface patterning and agrin treatment. J. Tissue Eng. Regen. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Hasirci, V. Mimicking corneal stroma using keratocyte-loaded photopolymerizable methacrylated gelatin hydrogels. J. Tissue Eng. Regen. Med. 2018, 12, e1899–e1910. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.M.; Shah, K.; Palani, S.; Peak, C.W.; Gaharwar, A.K. Gradient nanocomposite hydrogels for interface tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle-hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Y.; Xiao, W.; Chen, X.; Tang, Y.; Luo, H.; Fan, H. Bottom-up approach to build osteon-like structure by cell-laden photocrosslinkable hydrogel. Chem. Commun. 2012, 48, 3170–3172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tan, G.; Tan, Y.; Wang, H.; Liao, J.; Ning, C. Biomimetic mineralization of anionic gelatin hydrogels: Effect of degree of methacrylation. RSC Adv. 2014, 4, 21997–22008. [Google Scholar] [CrossRef]
- Xin, T.; Gu, Y.; Cheng, R.; Tang, J.; Sun, Z.; Cui, W.; Chen, L. Inorganic strengthened hydrogel membrane as regenerative periosteum. ACS Appl. Mater. Interfaces 2017, 9, 41168–41180. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. B 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Kawazoe, N.; Chen, G. Influence of microporous gelatin hydrogels on chondrocyte functions. J. Mater. Chem. B 2017, 5, 5753–5762. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, S.W.; Shridhar, S.V.; Zhang, K.; Albrecht, L.D.; Filip, A.B.; Horton, J.A.; Soman, P. Perfusion directed 3D mineral formation within cell-laden hydrogels. Biofabrication 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed]





| Tissue | Polymer | Cells | Aims & Achievements | Reference |
|---|---|---|---|---|
| Bone | GelMA−HA | HUVECs, MG63s | enhanced mechanical rigidity and cell functional expression, modularly engineering of biomimetic osteon. | [46] |
| GelMA-nSi | hMSCs | bone regeneration without any osteoinductive factors. | [47] | |
| GelMA-nHAp | preosteoblasts | increased mechanical stiffness and physiological stability, regeneration of the damaged bone tissue. | [48] | |
| Alginate-GelMA | MG63, HUVECs | simplified osteon mimicking. | [49] | |
| Gel/(nHAp-BMP-2) | BMSCs | increased organic/inorganic compatibility, sustainable bioactivity. | [50] | |
| GelMA | HUVECs, hMSCs | microstructured bone-like tissue constructs containing a perfusable vascular lumen. | [51] | |
| AlgMA-GelMA | MG63s, HUVECs | osteon-like structure by sequential assembly. | [52] | |
| GelMA/PEGDA | MC3T3-E1 | bone regeneration and enhanced performance. | [53] | |
| BG/GelMA | mBMSCs | improved bioactivity and stability of composites hydrogels. | [54] | |
| Bio-GelMA | ADSCs | cell carriers for ADSCs, greater osteogenic differentiation of ADSCs. | [55] | |
| GelMA | MSCs | vascularisation during endochondral bone repair. | [56] | |
| GelMA | BMSCs | injectable stem cell-Laden GelMA microspheres, rapid osteogenic Tissue Constructs. | [57] | |
| Cartilage | GelMA | meniscus fibrochondrocytes | combination of cell therapy, GelMA hydrogels, and PSL, production of graft tissue and emulation of meniscus collagen bundles. | [42] |
| GGMA/GelMA DNs | NIH-3T3 | cartilage mimicking. | [73] | |
| pMHMGCL/PCL- GelMA | chondrocytes | reinforced GelMA constructs. | [74] | |
| PEG-GelMA-HA | DPSCs | enhanced chondrogenesis in DPSCs. | [75] | |
| PEG-GelMA | hMSCs | enhanced mechanical strength. | [76] | |
| G-MeHA and G-MeCS | chondrocytes | phenotypic stability and integrated cartilage tissues. | [77] | |
| GelMA/PAM | chondrocytes | improved mechanical property and sustained release of growth factors. | [78] | |
| GelMA-HepSH | chondrocytes | promoted cell viability and chondrocyte phenotype. | [79] | |
| GelMA/gellan | chondrocytes | spatial position of chondrocytes in hydrogels and thus in defects. | [80] | |
| PEGDA/GelMA | MSCs | integration of living cells, biomaterials, and biological cues. | [81] | |
| endochondral bone | GelMA/CDM | MSCs | endochondral bone formation and relevant-size bone grafts. | [58] |
| GelMA/HAMA-ALG/HAP | chondrocytes | improved mechanical strength and chondrogenesis. | [59] | |
| vascular networks | GelMA | ECFCs and MSCs | generation of functional and 3D vascular networks. | [28] |
| GelMA | ECFCs and MSCs | photopolymerization in vitro and surgical transplant in vivo. | [82] | |
| PEG–GelMA | HUVECs | reproduction of the extracellular environment. | [83] | |
| GelMA | MC3T3 | fabrication of microchannel networks, promoted cellular viabilityand differentiation, and the formation of endothelial monolayers. | [84] | |
| GelMA | HUVECs | replicate of geometry and function of vascular networks and blood vessels. | [85] | |
| GelMA, sodium alginate, and PEGTA | ECs, MSCs | improved transport of oxygen, nutrients, and waste products. | [86] | |
| GelMA-alginate | ECs | generation of a scaffold with multilayer interlacing hydrogel microfibers | [87] | |
| GelMA | SCAP, HUVECs | photopolymerization under an LED-light source, and promoted vasculature network formation. | [88] | |
| GelMA/PCL | ECs | improved endothelium remodeling. | [89] | |
| skeletal muscle | GelMA | C2C12 | 3D arrays of engineered muscle tissue. | [90] |
| GelMA-CNT | C2C12 | anisotropic electrical conductivity and superior mechanical properties. | [91] | |
| GelMA | C2C12 | structures and the degree of alignment of myotubes. | [92] | |
| GelMA | C2C12 | enhanced myotube maturation and functionality. | [93] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. https://doi.org/10.3390/polym10111290
Sun M, Sun X, Wang Z, Guo S, Yu G, Yang H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers. 2018; 10(11):1290. https://doi.org/10.3390/polym10111290
Chicago/Turabian StyleSun, Mingyue, Xiaoting Sun, Ziyuan Wang, Shuyu Guo, Guangjiao Yu, and Huazhe Yang. 2018. "Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue" Polymers 10, no. 11: 1290. https://doi.org/10.3390/polym10111290