Sulfonated Binaphthyl-Containing Poly(arylene ether ketone)s with Rigid Backbone and Excellent Film-Forming Capability for Proton Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(arylene ether ketone)s (PAEKs)
2.3. Sulfonation of PAEKs
2.4. Membrane Preparation
2.5. Characterizations
3. Results and Discussion
3.1. Polymerization
3.2. Sulfonation
3.3. Ion Exchange Capacity of SPAEKs
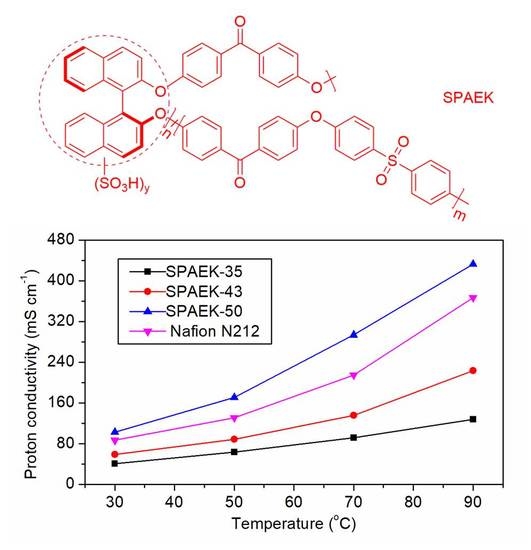
3.4. Water Affinity and Proton Conductivity of SPAEKs
3.5. Thermal Stability and Oxidative Stability of SPAEKs
3.6. Mechanical Properties of SPAEKs
3.7. Microscopic Morphologies of SPAEKs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vallejo, E.; Pourcelly, G.; Gavach, C.; Mercier, R.; Pineri, M. Sulfonated polyimides as proton conductor exchange membranes. Physicochemical properties and separation H+/Mz+ by electrodialysis comparison with a perfluorosulfonic membrane. J. Membr. Sci. 1999, 160, 127–137. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Lu, Y.Y.; Li, N. Preparation, characterization and performance of sulfonated poly (styrene-ethylene/butylene-styrene) block copolymer membranes for water desalination by pervaporation. Desalination 2016, 390, 33–46. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zhang, H.M.; Mai, Z.S.; Zhang, H.Z.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.W.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, F.; Ma, X.Y.; Guan, X.H.; Chen, D.Y.; Hickner, M.A. Sulfonated polymers containing polyhedral oligomeric silsesquioxane (POSS) core for high performance proton exchange membranes. Int. J. Hydrogen Energy 2015, 40, 7135–7143. [Google Scholar] [CrossRef]
- Aviles-Barreto, S.L.; Suleiman, L.D. Suleiman, Transport properties of sulfonated poly(styrene-isobutylene-styrene) membranes with counter-ion substitution. J. Appl. Polym. Sci. 2013, 129, 2294–2304. [Google Scholar] [CrossRef]
- Benavides, R.; Rodriguez, J.C.O.; Melo, L.; Morales-Acosta, D.; Paula, M.M.S.; da Silva, L. Electrochemical comparison of two sulfonated styrene PEM membranes synthesized by different methods. J. Appl. Electrochem. 2015, 45, 1211–1215. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Synthesis of poly(arylene ether ether ketone ketone) copolymers containing pendant sulfonic acid groups bonded to naphthalene as proton exchange membrane materials. Macromolecules 2004, 37, 6748–6754. [Google Scholar] [CrossRef]
- Guan, R.; Zou, H.; Lu, D.; Gong, C.; Liu, Y. Polyethersulfone sulfonated by chlorosulfonic acid and its membrane characteristics. Eur. Polym. J. 2005, 41, 1554–1560. [Google Scholar] [CrossRef]
- Wang, L.; Li, K.; Zhu, G.; Li, J. Preparation and properties of highly branched sulfonated poly(ether ether ketone)s doped with antioxidant 1010 as proton exchange membranes. J. Membr. Sci. 2011, 379, 440–448. [Google Scholar] [CrossRef]
- Li, G.B.; Zhao, C.J.; Li, X.F.; Qi, D.; Liu, C.; Bu, F.Z.; Na, H. Novel side-chain-type sulfonated diphenyl-based poly(arylene ether sulfone)s with a hydrogen-bonded network as proton exchange membranes. Polym. Chem. 2015, 6, 5911–5920. [Google Scholar] [CrossRef]
- Gui, L.; Zhang, C.; Kang, S.; Tan, N.; Xiao, G.; Yan, D. Synthesis and properties of hexafluoroisopropylidene-containing sulfonated poly(arylene thioether phosphine oxide)s for proton exchange membranes. Int. J. Hydrogen Energy 2010, 35, 2436–2445. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiao, M.; Meng, Y. Synthesis and characterization of novel sulfonated poly(arylene thioether) ionomers for vanadium redox flow battery applications. Energy Environ. Sci. 2010, 3, 622–628. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, Y.-B.; Bae, C. Partially fluorinated sulfonated poly(ether amide) fuel cell membranes: influence of chemical structure on membrane properties. Polymers 2011, 3, 222–235. [Google Scholar] [CrossRef]
- Li, N.W.; Cui, Z.M.; Zhang, S.B.; Xing, X. Sulfonated polyimides bearing benzimidazole groups for proton exchange membranes. Polymer 2007, 48, 7255–7263. [Google Scholar] [CrossRef]
- Ma, L.Y.; Xu, G.X.; Li, S.; Ma, J.; Li, J.; Cai, W.W. Design and optimization of a hyper-branched polyimide proton exchange membrane with ultra-high methanol-permeation resistivity for direct methanol fuel cells applications. Polymers 2018, 10, 1175. [Google Scholar] [CrossRef]
- Cui, M.B.; Zhang, Z.H.; Yuan, T.; Yang, H.; Wu, L.; Bakangura, E.; Xu, T.W. Proton-conducting membranes based on side-chain-type sulfonated poly(ether ketone/ether benzimidazole)s via one-pot condensation. J. Membr. Sci. 2014, 465, 100–106. [Google Scholar] [CrossRef]
- Jin, L.; Li, Z.; Wang, S.; Wang, Z.; Dong, F.; Yin, X. Highly conductive proton exchange membranes based on sulfonated poly(phthalazinone ether sulfone) and cerium sulfophenyl phosphate. React. Funct. Polym. 2012, 72, 549–555. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.H.; Jiang, Y.W.; Jian, X.G. Preparation and characterization of sulfonated poly(aryl ether ketone)s containing 3,5-diphenyl phthalazinone moieties for proton exchange membrane. RSC Adv. 2016, 6, 75328–75335. [Google Scholar] [CrossRef]
- Oh, K.; Ketpang, K.; Kim, H.; Shanmugam, S. Synthesis of sulfonated poly(arylene ether ketone) block copolymers for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 507, 135–142. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Yang, A.C.C.; Jankova, K.; Hvilsted, S.; Holdcroft, S. Enhancing the phase segregation and connectivity of hydrophilic channels by blending highly sulfonated graft copolymers with fluorous homopolymers. J. Mater. Chem. A 2016, 1, 8118–8126. [Google Scholar] [CrossRef]
- Yuk, J.; Lee, S.; Nugraha, A.F.; Lee, H.; Park, S.H.; Yi, S.D.; Bae, B. Synthesis and characterization of multi-block poly(arylene ether sulfone) membranes with highly sulfonated blocks for use in polymer electrolyte membrane fuel cells. J. Membr. Sci. 2016, 518, 50–59. [Google Scholar] [CrossRef]
- Sheng, L.; Higashihara, T.; Zakazawa, S.; Ueda, M. Polystyrenes containing flexible alkylsulfonated side chains as a proton exchange membrane for fuel cell application. Polym. Chem. 2012, 3, 3289–3295. [Google Scholar] [CrossRef]
- Shang, X.; Fang, S.; Meng, Y. Synthesis and characterization of poly(arylene ether ketone) with sulfonated fluorene pendants for proton exchange membrane. J. Membr. Sci. 2007, 297, 90–97. [Google Scholar] [CrossRef]
- Chang, Y.; Brunello, G.F.; Fuller, J.; Disabb-Miller, M.L.; Hawley, M.E.; Kim, Y.S.; Hickner, M.A.; Jang, S.S.; Bae, C. Polymer electrolyte membranes based on poly(arylene ether sulfone) with pendant perfluorosulfonic acid. Polym. Chem. 2013, 4, 272–281. [Google Scholar] [CrossRef]
- Lee, S.W.; Chen, J.C.; Wu, J.A.; Chen, K.H. Synthesis and properties of poly(ether sulfone)s with clustered sulfonic groups for PEMFC applications under various relative humidity. ACS Appl. Mater. Interfaces 2017, 9, 9805–9814. [Google Scholar] [CrossRef] [PubMed]
- Takamuku, S.; Jannasch, P. Multiblock copolymers with highly sulfonated blocks containing di-and tetrasulfonated arylene sulfone segments for proton exchange membrane fuel cell applications. Adv. Energy Mater. 2012, 2, 129–140. [Google Scholar] [CrossRef]
- Chen, G.; Pei, X.; Wei, H.; Xu, L.; Fang, X. Synthesis and characterization of sulfonated block copolyimides derived from 4,4′-sulfide-bis(naphthalic anhydride) for proton exchange membranes. J. Appl. Polym. Sci. 2015, 132, 41501. [Google Scholar] [CrossRef]
- Matsumoto, K.; Higashihara, T.; Ueda, M. Locally and densely sulfonated poly(ether sulfone)s as proton exchange membrane. Macromolecules 2009, 42, 1161–1166. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiao, M.; Meng, Y.; Hay, A. Novel polyaromatic ionomers with large hydrophilic domain and long hydrophobic chain targeting at highly proton conductive and stable membranes. J. Mater. Chem. 2011, 21, 12068–12077. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Hay, A.S. Ionomers for proton exchange membrane fuel cells with sulfonic acid groups on the end groups: Novel linear aromatic poly(sulfide-ketone)s. Macromolecules 2008, 41, 277–280. [Google Scholar] [CrossRef]
- Bae, B.; Miyatake, K.; Watanabe, M. Sulfonated poly(arylene ether sulfone ketone) multiblock copolymers with highly sulfonated block. Synthesis and properties. Macromolecules 2010, 43, 2684–2691. [Google Scholar] [CrossRef]
- Tian, M.; Pang, Z.B.; Li, H.F.; Wang, L.L. Novel MOP-type H-8-binaphthyl monodentate phosphite ligands and their applications in transition metal-catalyzed asymmetric 1,4-conjugate additions and hydroformylations. Tetrahedron Asymmetry 2017, 28, 330–337. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, P.; Jiang, H.C.; Wei, Y.; Shi, M. Chiral bidentate NHC ligands based on the 1,1’-binaphthyl scaffold: Synthesis and application in transition-metal-catalyzed asymmetric reactions. Chem. Rec. 2016, 16, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, S.; Xiao, M.; Meng, Y. Synthesis and properties of novel sulfonated poly(arylene ether sulfone) ionomers for vanadium redox flow battery. Energy Convers. Manag. 2010, 51, 2816–2824. [Google Scholar] [CrossRef]
- Yu, G.; Liu, C.; Wang, J.; Li, G.; Han, Y.; Jian, X. Synthesis, characterization, and crosslinking of soluble cyano-containing poly(arylene ether)s bearing phthalazinone moiety. Polymer 2010, 51, 100–109. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. Synthesis of sulfonated poly(fluorenyl ether thioether ketone)s with bulky-block structure and its application in vanadium redox flow battery. Polymer 2011, 52, 5312–5319. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Zhao, J.; Wang, S.; Wu, H.; Guiver, M.D.; Jiang, Z. Nanostructured ion exchange membranes for fuel cells: recent advances and perspectives. Adv. Mater. 2015, 27, 5280–5295. [Google Scholar] [CrossRef] [PubMed]
- Moh, L.C.H.; Goods, J.B.; Kim, Y.; Swager, T.M. Free volume enhanced proton exchange membranes from sulfonated triptycene poly(ether ketone). J. Membr. Sci. 2018, 549, 236–243. [Google Scholar] [CrossRef]
- Chen, X.; Lü, H.; Lin, Q.; Zhang, X.; Chen, D.; Zheng, Y. Partially fluorinated poly(arylene ether)s bearing long alkyl sulfonate side chains for stable and highly conductive proton exchange membranes. J. Membr. Sci. 2018, 549, 12–22. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. Ion clustering in quaternary ammonium functionalized benzylmethyl containing poly(arylene ether ketone)s. Macromolecules 2013, 46, 9270–9278. [Google Scholar] [CrossRef]











| Sample | Mn (kDa) | Mw (kDa) | Polydispersity Index |
|---|---|---|---|
| PAEK-35 | 42 | 67 | 1.6 |
| PEAK-43 | 35 | 58 | 1.7 |
| PAEK-50 | 51 | 76 | 1.5 |
| Sample | Theoretical IEC (mmol g−1) | Titrated IEC (mmol g−1) | SD (%) | Oxidative Stability (min.) |
|---|---|---|---|---|
| SPAEK-35 | 1.40 | 1.25 | 89 | 80 |
| SPAEK-43 | 1.65 | 1.63 | 99 | 75 |
| SPAEK-50 | 1.89 | 1.82 | 96 | 65 |
| Sample | Water Uptake (wt %) | Swelling Ratio (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| SPAEK-35 | 10.9 | 3.4 | 22.6 | 6.9 |
| SPAEK-43 | 12.3 | 4.2 | 17.8 | 19.9 |
| SPAEK-50 | 12.6 | 5.9 | 11.5 | 11.6 |
| N212 | 7.2 | 4.7 | 15.9 | 374.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, S.; Chen, D.; Ye, Z. Sulfonated Binaphthyl-Containing Poly(arylene ether ketone)s with Rigid Backbone and Excellent Film-Forming Capability for Proton Exchange Membranes. Polymers 2018, 10, 1287. https://doi.org/10.3390/polym10111287
Zhang W, Chen S, Chen D, Ye Z. Sulfonated Binaphthyl-Containing Poly(arylene ether ketone)s with Rigid Backbone and Excellent Film-Forming Capability for Proton Exchange Membranes. Polymers. 2018; 10(11):1287. https://doi.org/10.3390/polym10111287
Chicago/Turabian StyleZhang, Wenmeng, Shaoyun Chen, Dongyang Chen, and Zhuoliang Ye. 2018. "Sulfonated Binaphthyl-Containing Poly(arylene ether ketone)s with Rigid Backbone and Excellent Film-Forming Capability for Proton Exchange Membranes" Polymers 10, no. 11: 1287. https://doi.org/10.3390/polym10111287