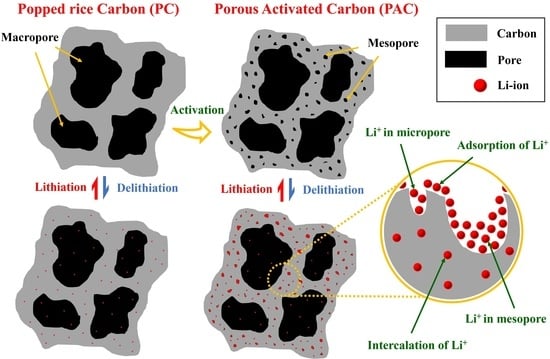
Natural Porous Carbon Derived from Popped Rice as Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterizations
2.3. Electrochemical Measurements
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, A.D.; Li, X.; Zhang, H. Porous Carbon Spheres and Monoliths: Morphology Control, Pore Size Tuning and Their Applications as Li-Ion Battery Anode Materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Zhao, Y.; Xia, Y.; Ouyang, M.; Brandon, N.; Banerjee, A. Revisiting the Promise of Bi-Layer Graded Cathodes for Improved Li-Ion Battery Performance. Sustain. Energy Fuels 2021, 5, 5193–5204. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Namsar, O.; Autthawong, T.; Laokawee, V.; Boonprachai, R.; Haruta, M.; Kurata, H.; Yu, A.; Chairuangsri, T.; Sarakonsri, T. Improved Electrochemical Performance of Anode Materials for High Energy Density Lithium-Ion Batteries through Sn(SnO2)–SiO2/Graphene-Based Nanocomposites Prepared by a Facile and Low-Cost Approach. Sustain. Energy Fuels 2020, 4, 4625–4636. [Google Scholar] [CrossRef]
- Chen, L.-F.; Ma, S.-X.; Lu, S.; Feng, Y.; Zhang, J.; Xin, S.; Yu, S.-H. Biotemplated Synthesis of Three-Dimensional Porous MnO/C-N Nanocomposites from Renewable Rapeseed Pollen: An Anode Material for Lithium-Ion Batteries. Nano Res. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material—Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated Amorphous Carbon with High-Porosity Derived From Camellia Pollen Grains as Anode Materials for Lithium/Sodium Ion Batteries. Front. Chem. 2018, 6, 366. [Google Scholar] [CrossRef]
- Kim, T.; Jo, C.; Lim, W.-G.; Lee, J.; Lee, J.; Lee, K.-H. Facile Conversion of Activated Carbon to Battery Anode Material Using Microwave Graphitization. Carbon 2016, 104, 106–111. [Google Scholar] [CrossRef]
- Lei, H.; Wang, Y.; Huo, J. Porous Graphitic Carbon Materials Prepared from Cornstarch with the Assistance of Microwave Irradiation. Microporous Mesoporous Mater. 2015, 210, 39–45. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.J.; Ulrich, S.; Araujo, P.T.; Bakker, M.G. Catalytic Graphitization in Nanocast Carbon Monoliths by Iron, Cobalt and Nickel Nanoparticles. Carbon 2018, 134, 452–463. [Google Scholar] [CrossRef]
- Zhao, L.; Bennett, J.C.; Obrovac, M.N. Hexagonal Platelet Graphite and Its Application in Li-Ion Batteries. Carbon 2018, 134, 507–518. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wang, X.; Liu, W.; Liang, J.; Liang, C. Preparation of Porous Carbon Anode Materials for Lithium-Ion Battery from Rice Husk. Mater. Lett. 2019, 253, 405–408. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Gao, B.; Li, X.; Cai, Q.; Li, Q.; Peng, X.; Huo, K.; Chu, P.K. Hierarchical Porous Carbon Materials Derived from Self-Template Bamboo Leaves for Lithium–Sulfur Batteries. Electrochim. Acta 2017, 229, 352–360. [Google Scholar] [CrossRef]
- Tang, J.; Etacheri, V.; Pol, V.G. From Allergens to Battery Anodes: Nature-Inspired, Pollen Derived Carbon Architectures for Room- and Elevated- Temperature Li-Ion Storage. Sci. Rep. 2016, 6, 20290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.-S.; Titirici, M.-M.; Chen, L.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave Pyrolysis of Lignocellulosic Biomass: Heating Performance and Reaction Kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Wang, R.; Lou, M.; Zhang, J.; Sun, Z.; Li, Z.; Wen, P. ZIF-8@ZIF-67-Derived Co Embedded into Nitrogen-Doped Carbon Nanotube Hollow Porous Carbon Supported Pt as an Efficient Electrocatalyst for Methanol Oxidation. Nanomaterials 2021, 11, 2491. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Carbonaceous Anode Materials for Non-aqueous Sodium- and Potassium-Ion Hybrid Capacitors. ACS Energy Lett. 2021, 6, 4127–4154. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural Engineering and Surface Modification of MOF-Derived Cobalt-Based Hybrid Nanosheets for Flexible Solid-State Supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Reis, G.S.D.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable Biomass Activated Carbons as Electrodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from Biomass Precursors as Anode Materials for Lithium Ion Batteries: New Insights into Carbonization and Graphitization Behavior and into Their Correlation to Electrochemical Performance. Carbon 2017, 128, 147–163. [Google Scholar] [CrossRef]
- Kumar, U.; Wu, J.; Sharma, N.; Sahajwalla, V. Biomass Derived High Areal and Specific Capacity Hard Carbon Anodes for Sodium-Ion Batteries. Energy Fuels 2021, 35, 1820–1830. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zheng, J.; Mu, Y.; Jiang, H.; Yan, H.; Wang, Y.; Zhang, Y.; Meng, C. Rice-like and Rose-like Zinc Silicates Anchored on Amorphous Carbon Derived from Natural Reed Leaves for High-Performance Supercapacitors. Dalt. Trans. 2021, 50, 9438–9449. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, X.; Zhang, Q.; Tu, J. Popcorn Inspired Porous Macrocellular Carbon: Rapid Puffing Fabrication from Rice and Its Applications in Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1701110. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-Derived Mesopore-Dominant Porous Carbons with Large Specific Surface Area and High Defect Density as High Performance Electrode Materials for Li-Ion Batteries and Supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Yu, C.; Chen, M.; Li, X.; Zhao, C.; He, L.; Qiu, J. Hierarchically Porous Carbon Architectures Embedded with Hollow Nanocapsules for High-Performance Lithium Storage. J. Mater. Chem. A 2015, 3, 5054–5059. [Google Scholar] [CrossRef]
- Cesano, F.; Cravanzola, S.; Brunella, V.; Scarano, D. Porous Carbon Spheres from Poly(4-Ethylstyrene-Co-Divinylbenzene: Role of ZnCl2 and KOH Agents in Affecting Porosity, Surface Area and Mechanical Properties. Microporous Mesoporous Mater. 2019, 288, 109605. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave Assisted Preparation of Activated Carbon from Biomass: A Review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Optimization of Preparation Conditions for Activated Carbons from Date Stones Using Response Surface Methodology. Powder Technol. 2012, 224, 101–108. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, K.; Tahir, M.; Wu, X.; Idrees, F.; Shen, M.; Cao, C. Tunable Porous Structure of Carbon Nanosheets Derived from Puffed Rice for High Energy Density Supercapacitors. J. Power Sources 2017, 371, 148–155. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Sun, Z.; Zhang, D.; Huang, Y.; Gu, S.; Chen, W. Understanding Reactions and Pore-Forming Mechanisms between Waste Cotton Woven and FeCl3 during the Synthesis of Magnetic Activated Carbon. Chemosphere 2020, 241, 125120. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, X.; Hassan, E.B.; Wang, C.; Zhang, J.; Cai, Z. Catalytic Graphitization of Kraft Lignin to Graphene-Based Structures with Four Different Transitional Metals. J. Nanoparticle Res. 2018, 20, 223. [Google Scholar] [CrossRef]
- Xia, S.; Cai, N.; Wu, J.; Xiao, H.; Hu, J.; Chen, X.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Synthesis and Formation Mechanism of Biomass-Based Mesoporous Graphitic Carbon. Fuel Process. Technol. 2020, 209, 106543. [Google Scholar] [CrossRef]
- Wang, Y.; Serrano, S.; Santiago-Avilés, J.J. Raman Characterization of Carbon Nanofibers Prepared Using Electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
- Mori, F.; Kubouchi, M.; Arao, Y. Effect of Graphite Structures on the Productivity and Quality of Few-Layer Graphene in Liquid-Phase Exfoliation. J. Mater. Sci. 2018, 53, 12807–12815. [Google Scholar] [CrossRef]
- Tai, Z.; Zhang, Q.; Liu, Y.; Liu, H.; Dou, S. Activated Carbon from the Graphite with Increased Rate Capability for the Potassium Ion Battery. Carbon 2017, 123, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Tallant, D.; Friedmann, T.; Missert, N.; Siegal, M.; Sullivan, J. Raman Spectroscopy of Amorphous Carbon. MRS Proc. 1998, 498, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wu, Y.; Choong, C.K.S.; Zhang, J.; Teo, K.L.; Ni, Z.; Shen, Z. Disorder Induced Bands in First Order Raman Spectra of Carbon Nanowalls. In Proceedings of the Sixth IEEE Conference on Nanotechnology, Cincinnati, OH, USA, 17–20 June 2006; Volume 1, pp. 219–222. [Google Scholar]
- Sing, K.S.W. Reporting Physisorption Data for Gas/solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Zhang, K.; Yu, X.; Yuan, J.; Zhu, D.-M.; Ozawa, K.; Qin, L.-C. Effect of Porous Structural Properties on Lithium-Ion and Sodium-Ion Storage: Illustrated by the Example of a Micro-Mesoporous Graphene1−x(MoS2)x Anode. RSC Adv. 2021, 11, 34152–34159. [Google Scholar] [CrossRef]
- Lemoisson, F.; Froyen, L. 12—Understanding and Improving Powder Metallurgical Processes. In Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Cambridge, UK, 2005; pp. 471–502. [Google Scholar]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of Activated Carbons Prepared from Wheat Straw via ZnCl2 and KOH Activation. Waste Biomass Valorization 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. C J. Carbon Res. 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Ostadhassan, M. The Impact of Pore Size Distribution Data Presentation Format on Pore Structure Interpretation of Shales. Adv. Geo-Energy Res. 2019, 3, 187–197. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, S.; Hwang, J. Graphene-Based Two-Dimensional Mesoporous Materials: Synthesis and Electrochemical Energy Storage Applications. Materials 2021, 14, 2597. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, X.; Guan, Z.; Tong, Y.; Xu, B.; Wang, X.; Wang, Z.; Chen, L. Insights into Lithium and Sodium Storage in Porous Carbon. Nano Lett. 2020, 20, 3836–3843. [Google Scholar] [CrossRef]
- Joho, F.; Rykart, B.; Blome, A.; Novák, P.; Wilhelm, H.; Spahr, M.E. Relation between Surface Properties, Pore Structure and First-Cycle Charge Loss of Graphite as Negative Electrode in Lithium-Ion Batteries. J. Power Sources 2001, 97–98, 78–82. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, H.; Qi, H.; Liang, J.; Liang, C. High Performance of Porous Silicon/Carbon/RGO Network Derived from Rice Husks as Anodes for Lithium-Ion Batteries. New J. Chem. 2018, 42, 19811–19817. [Google Scholar] [CrossRef]
- Gan, L.; Guo, H.; Wang, Z.; Li, X.; Peng, W.; Wang, J.; Huang, S.; Su, M. A Facile Synthesis of Graphite/Silicon/Graphene Spherical Composite Anode for Lithium-Ion Batteries. Electrochim. Acta 2013, 104, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Mu, L.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Pitch-Derived Amorphous Carbon as High Performance Anode for Sodium-Ion Batteries. Energy Storage Mater. 2016, 2, 139–145. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An Urgent Call to Spent LIB Recycling: Whys and Wherefores for Graphite Recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Velez, V.; Ramos-Sánchez, G.; Lopez, B.; Lartundo-Rojas, L.; González, I.; Sierra, L. Synthesis of Novel Hard Mesoporous Carbons and Their Applications as Anodes for Li and Na Ion Batteries. Carbon 2019, 147, 214–226. [Google Scholar] [CrossRef]
- Um, J.H.; Choi, M.; Park, H.; Cho, Y.-H.; Dunand, D.C.; Choe, H.; Sung, Y.-E. 3D Macroporous Electrode and High-Performance in Lithium-Ion Batteries Using SnO2 Coated on Cu Foam. Sci. Rep. 2016, 6, 18626. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-K.; Li, C.-Y.V.; Deng, Z.; Chan, K.-Y.; Yung, H.; Yang, C. Hierarchical Macropore-Mesoporous Shell Carbon Dispersed with Li4Ti5O12 for Excellent High Rate Sub-Freezing Li-Ion Battery Performance. Carbon N. Y. 2019, 145, 614–621. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, Y.; Qian, J.; Ai, X.; Yang, H. Antimony-Coated SiC Nanoparticles as Stable and High-Capacity Anode Materials for Li-Ion Batteries. J. Phys. Chem. C 2010, 114, 15196–15201. [Google Scholar] [CrossRef]
- Kim, J.; Nithya Jeghan, S.M.; Lee, G. Superior Fast-Charging Capability of Graphite Anode via Facile Surface Treatment for Lithium-Ion Batteries. Microporous Mesoporous Mater. 2020, 305, 110325. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.-S.; Aravindan, V. Achieving High-Energy Dual Carbon Li-Ion Capacitors with Unique Low- and High-Temperature Performance from Spent Li-Ion Batteries. J. Mater. Chem. A 2020, 8, 4950–4959. [Google Scholar] [CrossRef]
- Shi, J.; Lin, N.; Liu, D.; Wang, Y.; Lin, H. Preparation of C/SnO2 Composite with Rice Husk-Based Porous Carbon Carrier Loading Ultrasmall SnO2 Nanoparticles for Anode in Lithium-Ion Batteries. J. Electroanal. Chem. 2020, 857, 113634. [Google Scholar] [CrossRef]
- Xiao, Y.; Cao, M.; Ren, L.; Hu, C. Hierarchically Porous Germanium-Modified Carbon Materials with Enhanced Lithium Storage Performance. Nanoscale 2012, 4, 7469–7474. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, K.; Zhang, L.; Wang, C. N-Doped 3D Porous Carbon Material Derived from Hierarchical Porous IRMOF-3 Using Citric Acid Modulator: Fabrication and Application in LIBs as the Anode Materials. Dalt. Trans. 2020, 49, 9369–9376. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.; Yang, X.; Zheng, Y.; Liu, C.; Di, M.; Zheng, Z. Flexible Anode Materials for Lithium-Ion Batteries Derived from Waste Biomass-Based Carbon Nanofibers: I. Effect of Carbonization Temperature. RSC Adv. 2018, 8, 7102–7109. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Liu, T.; Zhang, J.; Zhang, L.; Liu, Y.; Huang, J.; Guo, S. Sweet Potato-Derived Carbon Nanoparticles as Anode for Lithium Ion Battery. RSC Adv. 2015, 5, 40737–40741. [Google Scholar] [CrossRef]
- Yokokura, T.J.; Rodriguez, J.R.; Pol, V.G. Waste Biomass-Derived Carbon Anode for Enhanced Lithium Storage. ACS Omega 2020, 5, 19715–19720. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Adams, R.A.; Kim, P.J.; Arora, A.; Martinez, E.; Youngblood, J.P.; Pol, V.G. Li-Ion Storage in an Amorphous, Solid, Spheroidal Carbon Anode Produced by Dry-Autoclaving of Coffee Oil. Carbon N. Y. 2018, 133, 62–68. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Li, Y.; Zhang, X.; Hardin, I.R. Graphene-Coated Pyrogenic Carbon as an Anode Material for Lithium Battery. Chem. Eng. J. 2013, 229, 399–403. [Google Scholar] [CrossRef]




| Materials | Surface and Pore Parameters | Discharge Capacity (mAh g−1) | ||||
|---|---|---|---|---|---|---|
| Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore (nm) | 1st Cycle | 2nd Cycle | 150th Cycle | |
| Non-activated PC | 54.56 | 0.0720 | 2.639 | 842 | 293 | 189 |
| PAC-KOH | 1695.58 | 0.9442 | 1.116 | 2008 | 759 | 383 |
| PAC-ZnCl2 | 1677.66 | 0.9364 | 1.114 | 911 | 392 | 370 |
| PAC-FeCl3 | 732.62 | 0.3725 | 1.017 | 1004 | 373 | 320 |
| PAC-Mg | 46.34 | 0.1119 | 4.831 | 356 | 280 | 252 |
| Materials | Capacity (mAh g−1) | Cycle Number | Current Density (mA g−1) | Ref. |
|---|---|---|---|---|
| Non-activated PC | 189 | 150 | 100 | This work |
| PAC-KOH | 383 | 150 | 100 | This work |
| Pristine graphite | ~290 | 50 | 100 | [57] |
| Recovered graphite | ~280 | 200 | 100 | [58] |
| Porous carbon | ~330 | 50 | 100 | [59] |
| Pure carbon | 248.9 | 30 | 74.4 (0.2C) | [60] |
| N-doped 3D porous carbon (at 1000 °C) | 224 | 100 | 200 | [61] |
| Carbon nanofibers (at 1000 °C) | 239.6 | 200 | 100 | [62] |
| Carbon nanoparticles | 320 | 200 | 100 | [63] |
| Waste avocado seeds-derived carbon | 315 | 100 | 100 | [64] |
| Spheroidal carbon | 342 | 100 | 100 | [65] |
| Graphene-coated pyrogenic carbon | 288 | 50 | 50 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonprachai, R.; Autthawong, T.; Namsar, O.; Yodbunork, C.; Yodying, W.; Sarakonsri, T. Natural Porous Carbon Derived from Popped Rice as Anode Materials for Lithium-Ion Batteries. Crystals 2022, 12, 223. https://doi.org/10.3390/cryst12020223
Boonprachai R, Autthawong T, Namsar O, Yodbunork C, Yodying W, Sarakonsri T. Natural Porous Carbon Derived from Popped Rice as Anode Materials for Lithium-Ion Batteries. Crystals. 2022; 12(2):223. https://doi.org/10.3390/cryst12020223
Chicago/Turabian StyleBoonprachai, Ruttapol, Thanapat Autthawong, Orapim Namsar, Chawin Yodbunork, Waewwow Yodying, and Thapanee Sarakonsri. 2022. "Natural Porous Carbon Derived from Popped Rice as Anode Materials for Lithium-Ion Batteries" Crystals 12, no. 2: 223. https://doi.org/10.3390/cryst12020223