Surface Passivation Using N-Type Organic Semiconductor by One-Step Method in Two-Dimensional Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Device Fabrication
2.3. Characterization
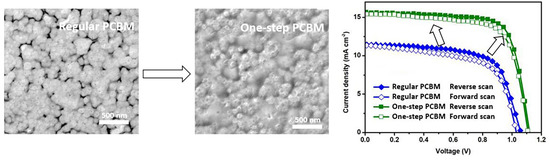
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Yang, S.; Cao, B.; Tao, X.; Chen, Z. Single crystal perovskite solar cells: Development and perspectives. Adv. Funct. Mater. 2019, 30, 1905021. [Google Scholar] [CrossRef]
- Huang, F.; Siffalovic, P.; Li, B.; Yang, S.; Zhang, L.; Nadazdy, P.; Cao, G.; Tian, J. Controlled crystallinity and morphologies of 2D Ruddlesden-Popper perovskite films grown without anti-solvent for solar cells. Chem. Eng. J. 2020, 394, 124959. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Lee, C.-L.; Kim, G.; Kim, T.K.; Back, H.; Jung, S.; Yu, K.; Hong, S.; Lee, S.; et al. A printable organic electron transport layer for low-temperature-processed, hysteresis-free, and stable planar perovskite solar cells. Adv. Energy Mater. 2017, 7, 1700226. [Google Scholar] [CrossRef]
- Wang, T.; Scarratt, N.W.; Yi, H.; Dunbar, A.; Pearson, A.; Watters, D.C.; Glen, T.; Brook, A.C.; Kingsley, J.; Buckley, A.R.; et al. Fabricating high performance, donor-acceptor copolymer solar cells by spray-coating in air. Adv. Energy Mater. 2013, 3, 505–512. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, E.; Shao, Y.; Xiao, Z.; Dong, Q.; Huang, J. Scalable fabrication of efficient organolead trihalide perovskite solar cells with doctor-bladed active layers. Energy Environ. Sci. 2015, 8, 1544–1550. [Google Scholar] [CrossRef]
- Bishop, J.E.; Routledge, T.J.; Lidzey, D.G. Advances in spray-cast perovskite solar cells. J. Phys. Chem. Lett. 2018, 9, 1977–1984. [Google Scholar] [CrossRef]
- Huang, H.; Shi, J.; Zhu, L.; Li, D.; Luo, Y.; Meng, Q. Two-step ultrasonic spray deposition of CH3NH3PbI3 for efficient and large-area perovskite solar cell. Nano Energy 2016, 27, 352–358. [Google Scholar] [CrossRef]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on perovskite materials and solar cells with mixed cations and halide anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-efficiency rubidium-incorporated perovskite solar cells by gas quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Jacobs, R.; Luo, G.; Morgan, D. Materials discovery of stable and nontoxic halide perovskite materials for high-efficiency solar cells. Adv. Funct. Mater. 2019, 29, 1804354. [Google Scholar] [CrossRef] [Green Version]
- Grandhi, G.; Matuhina, A.; Liu, M.; Annurakshita, S.; Ali-Löytty, H.; Bautista, G.; Vivo, P. Lead-free cesium titanium bromide double perovskite nanocrystals. Nanomaterials 2021, 11, 1458. [Google Scholar] [CrossRef]
- Li, X.; Zeng, P.; Ou, Q.; Zhang, S. Fabrication of perovskite film-coated hollow capillary fibers using a fast solvent exchange method. Nanomaterials 2021, 11, 1483. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Lee, E.-C. High-performance metal oxide-free inverted perovskite solar cells using poly (bis (4-phenyl)(2, 4, 6-trimethylphenyl) amine) as the hole transport layer. J. Mater. Chem. C 2018, 6, 6975–6981. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Lee, E.-C. High-performance inverted planar perovskite solar cells using a pristine fullerene mixture as an electron-transport layer. J. Mater. Chem. C 2019, 7, 6956–6963. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Lee, E.-C. Crystallization management for high-performance perovskite solar cells by introducing antisolvent into perovskite precursor. J. Mater. Chem. C 2020, 8, 15860–15867. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Lee, E.-C. Non-equivalent Tl doping for high performance perovskite solar cells: Crystal quality improvement with enhanced p-type character. J. Power Sources 2020, 479, 228818. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, H.; Tong, J.; Berry, J.J.; Beard, M.C.; Zhu, K. Advances in two-dimensional organic-inorganic hybrid perovskites. Energy Environ. Sci. 2020, 13, 1154–1186. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Liu, B.; Munir, R.; Zhu, X.; Yang, D.; Li, J.; Liu, Y.; Smilgies, D.-M.; Li, R.; et al. Stable high efficiency two-dimensional perovskite solar cells via cesium doping. Energy Environ. Sci. 2017, 10, 2095–2102. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Z.; Zuo, S.; Feng, J.; Wang, Z.; Zhang, X.; Zhao, K.; Zhang, J.; Liu, H.; Priya, S.; et al. Vapor-fumigation for record efficiency two-dimensional perovskite solar cells with superior stability. Energy Environ. Sci. 2018, 11, 3349–3357. [Google Scholar] [CrossRef]
- Cao, D.H.; Stoumpos, C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, Y.; Li, L.; Tan, S.; Liu, N.; Zheng, G.; Chen, Q.; Zhou, H. Exploration of crystallization kinetics in quasi two-dimensional perovskite and high performance solar cells. J. Am. Chem. Soc. 2018, 140, 459–465. [Google Scholar] [CrossRef]
- Etgar, L. The merit of perovskite’s dimensionality; can this replace the 3D halide perovskite? Energy Environ. Sci. 2018, 11, 234–242. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Sun, Y.; Liang, Z. Phase engineering in quasi-2D ruddlesden-popper perovskites. J. Phys. Chem. Lett. 2018, 9, 2627–2631. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.-C.; Stoumpos, C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef]
- Ren, H.; Yu, S.; Chao, L.; Xia, Y.; Sun, Y.; Zuo, S.; Li, F.; Niu, T.; Yang, Y.; Ju, H.; et al. Efficient and stable Ruddlesden–Popper perovskite solar cell with tailored interlayer molecular interaction. Nat. Photon. 2020, 14, 154–163. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Fu, W.; Qin, M.; Yang, W.; Yan, J.; Zhang, Z.; Lu, X.; Chen, H. Orientation regulation of Phenylethylammonium cation based 2D perovskite solar cell with efficiency higher than 11%. Adv. Energy Mater. 2018, 8, 1702498. [Google Scholar] [CrossRef]
- Zhou, L.; Chang, J.; Liu, Z.; Sun, X.; Lin, Z.; Chen, D.; Zhang, C.; Zhang, J.; Hao, Y. Enhanced planar perovskite solar cell efficiency and stability using a perovskite/PCBM heterojunction formed in one step. Nanoscale 2018, 10, 3053–3059. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat. Energy 2016, 1, 15001. [Google Scholar] [CrossRef]
- Wolff, C.M.; Zu, F.; Paulke, A.; Toro, L.P.; Koch, N.; Neher, D. Reduced interface-mediated recombination for high open-circuit voltages in CH3 NH3 PbI3 solar cells. Adv. Mater. 2017, 29, 1700159. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-W.; Chueh, C.-C.; Williams, S.T.; Jen, A.K.-Y. Roles of fullerene-based interlayers in enhancing the performance of organometal perovskite thin-film solar cells. Adv. Energy Mater. 2015, 5, 1402321. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Huang, W.-K.; Chang, Y.-C.; Lee, K.-T.; Chen, C.-T. A solution-processed n-doped fullerene cathode interfacial layer for efficient and stable large-area perovskite solar cells. J. Mater. Chem. A 2016, 4, 640–648. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y.; Li, Y. Fullerene derivatives for the applications as acceptor and cathode buffer layer materials for organic and perovskite solar cells. Adv. Energy Mater. 2017, 7, 1601251. [Google Scholar] [CrossRef]
- Xia, F.; Wu, Q.; Zhou, P.; Li, Y.; Chen, X.; Liu, Q.; Zhu, J.; Dai, S.; Lu, Y.; Yang, S. efficiency enhancement of inverted structure perovskite solar cells via oleamide doping of PCBM electron transport layer. ACS Appl. Mater. Interfaces 2015, 7, 13659–13665. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, G.; Xu, C.; Zeng, F.; Xie, X.; Wu, S. Surface Passivation Using N-Type Organic Semiconductor by One-Step Method in Two-Dimensional Perovskite Solar Cells. Crystals 2021, 11, 933. https://doi.org/10.3390/cryst11080933
Wang H, Liu G, Xu C, Zeng F, Xie X, Wu S. Surface Passivation Using N-Type Organic Semiconductor by One-Step Method in Two-Dimensional Perovskite Solar Cells. Crystals. 2021; 11(8):933. https://doi.org/10.3390/cryst11080933
Chicago/Turabian StyleWang, Helong, Guanchen Liu, Chongyang Xu, Fanming Zeng, Xiaoyin Xie, and Sheng Wu. 2021. "Surface Passivation Using N-Type Organic Semiconductor by One-Step Method in Two-Dimensional Perovskite Solar Cells" Crystals 11, no. 8: 933. https://doi.org/10.3390/cryst11080933