Lead-Free Perovskite Single Crystals: A Brief Review
Abstract
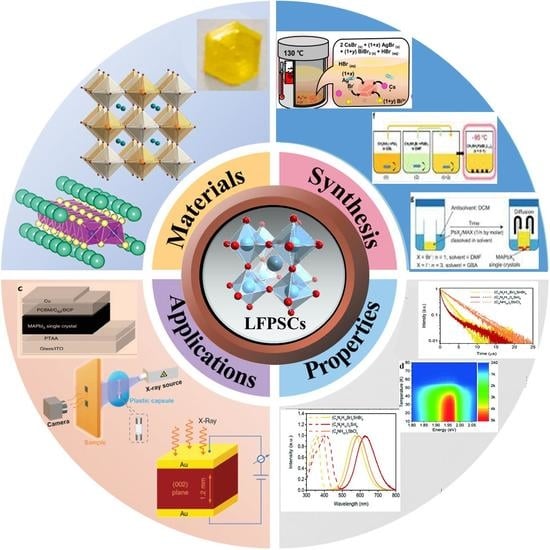
:1. Introduction
2. Various Systems of Pb-Free Single Crystal
2.1. Sn Based Halide Perovskites
2.2. Bi/Sb Based Halide Perovskites
2.3. Other Metals Based Perovskites
2.4. Halide Double Perovskites
3. Applications
3.1. Photodetectors
3.2. Solar Cells
3.3. X-ray Detectors
3.4. Light-Emitting Diodes
3.5. Humidity Sensor and Field-Effect Transistors
4. Challenges and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Hazarika, A.; Chen, X.; Harvey, S.P.; Larson, B.W.; Teeter, G.R.; Liu, J.; Song, T.; Xiao, C.; Shaw, L.; et al. High efficiency perovskite quantum dot solar cells with charge separating heterostructure. Nat. Commun. 2019, 10, 2842. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, D.H. Perovskite-based photodetectors: Materials and devices. Chem. Soc. Rev. 2017, 46, 5204–5236. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for alpha-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yang, Z.; Zhou, N.; Deng, Y.; Zhao, J.; Xiao, X.; Rudd, P.; Moran, A.; Yan, Y.; et al. Efficient sky-blue perovskite light-emitting diodes via photoluminescence enhancement. Nat. Commun. 2019, 10, 5633. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Luo, J.; Hu, M.; Niu, G.; Tang, J. Lead-free halide perovskites and perovskite variants as phosphors toward light-emitting applications. ACS Appl. Mater. Interfaces 2019, 11, 31575–31584. [Google Scholar] [CrossRef]
- Bhaumik, S.; Ray, S.; Batabyal, S.K. Recent advances of lead-free metal halide perovskite single crystals and nanocrystals: Synthesis, crystal structure, optical properties, and their diverse applications. Mater. Today Chem. 2020, 18, 100363. [Google Scholar] [CrossRef]
- Jiang, H.; Kloc, C. Single-crystal growth of organic semiconductors. MRS Bull. 2013, 38, 28–33. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, Z.; Huang, J. Light-Induced Self-Poling Effect on Organometal Trihalide Perovskite Solar Cells for Increased Device Efficiency and Stability. Adv. Energy Mater. 2015, 5, 1500721. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Raino, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bi, W.; Wang, A.; Liu, X.; Kang, Y.; Dong, Q. Efficient lateral-structure perovskite single crystal solar cells with high operational stability. Nat. Commun. 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, L.; Cheng, X.; Yuan, Y.; Du, S.; Ding, J.; Sun, H.; Zhan, X.; Zhou, T. Design Growth of Triangular Pyramid MAPbBr3 Single Crystal and Its Photoelectric Anisotropy between (100) and (111) Facets. J. Phys. Chem. C 2019, 123, 10826–10830. [Google Scholar] [CrossRef]
- Yang, C.; El-Demellawi, J.K.; Yin, J.; Velusamy, D.B.; Emwas, A.-H.M.; El-Zohry, A.M.; Gereige, I.; AlSaggaf, A.; Bakr, O.M.; Alshareef, H.N.; et al. MAPbI3 Single Crystals Free from Hole-Trapping Centers for Enhanced Photodetectivity. ACS Energy Lett. 2019, 4, 2579–2584. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Peng, J.; Sun, Y.; Liang, Z. Structure and Growth Control of Organic-Inorganic Halide Perovskites for Optoelectronics: From Polycrystalline Films to Single Crystals. Adv. Sci. 2016, 3, 1500392. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Xie, W.; Mathews, N.; Sherburne, M.; Ahuja, R.; Asta, M.; Mhaisalkar, S.G. Rational Design: A High-Throughput Computational Screening and Experimental Validation Methodology for Lead-Free and Emergent Hybrid Perovskites. ACS Energy Lett. 2017, 2, 837–845. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Tailor, N.K.; Kar, S.; Mishra, P.; These, A.; Kupfer, C.; Hu, H.; Awais, M.; Saidaminov, M.; Dar, M.I.; Brabec, C.; et al. Advances in Lead-Free Perovskite Single Crystals: Fundamentals and Applications. ACS Mater. Lett. 2021, 3, 1025–1080. [Google Scholar] [CrossRef]
- Zhao, S.; Cai, W.; Wang, H.; Zang, Z.; Chen, J. All-Inorganic Lead-Free Perovskite(-Like) Single Crystals: Synthesis, Properties, and Applications. Small Methods 2021, 5, 2001308. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What defines a halide perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaife, D.E.; Weller, P.F.; Fisher, W.G. Crystal preparation and properties of cesium tin (II) trihalides. J. Solid State Chem. 1974, 9, 308–314. [Google Scholar] [CrossRef]
- Chung, I.; Song, J.-H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G. CsSnI3: Semiconductor or metal? High electrical conductivity and strong near-infrared photoluminescence from a single material. High hole mobility and phase-transitions. J. Am. Chem. Soc. 2012, 134, 8579–8587. [Google Scholar] [CrossRef] [PubMed]
- Kahmann, S.; Nazarenko, O.; Shao, S.; Hordiichuk, O.; Kepenekian, M.; Even, J.; Kovalenko, M.V.; Blake, G.R.; Loi, M.A. Negative Thermal Quenching in FASnI3 Perovskite Single Crystals and Thin Films. ACS Energy Lett. 2020, 5, 2512–2519. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, Z.; Liu, Y.; Zhao, W.; Zhang, X.; Liu, B.; Wu, H.; Liu, S. Local temperature reduction induced crystallization of MASnI3 and achieving a direct wafer production. RSC Adv. 2017, 7, 38155–38159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Mao, X.; Cheng, P.; Yang, Y.; Yang, S.; Wumaier, T.; Deng, W.; Han, K. Bismuth doped lead-free two-dimensional tin based halide perovskite single crystals. J. Energy Chem. 2019, 36, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lehner, A.J.; Fabini, D.H.; Evans, H.A.; Hébert, C.-A.; Smock, S.R.; Hu, J.; Wang, H.; Zwanziger, J.W.; Chabinyc, M.L.; Seshadri, R. Crystal and Electronic Structures of Complex Bismuth Iodides A3Bi2I9 (A = K, Rb, Cs) Related to Perovskite: Aiding the Rational Design of Photovoltaics. Chem. Mater. 2015, 27, 7137–7148. [Google Scholar] [CrossRef] [Green Version]
- McCall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron–Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- McCall, K.M.; Liu, Z.; Trimarchi, G.; Stoumpos, C.C.; Lin, W.; He, Y.; Hadar, I.; Kanatzidis, M.G.; Wessels, B.W. α-Particle Detection and Charge Transport Characteristics in the A3M2I9Defect Perovskites (A = Cs, Rb; M = Bi, Sb). ACS Photonics 2018, 5, 3748–3762. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.; Huang, Z.; Wei, J.; Wang, X.; Li, W.; Chen, H.; Kuang, D.; Su, C. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, J.; Huang, Z.; Wei, J.; Wang, X.; Chen, H.; Kuang, D. Intrinsic Self-Trapped Emission in 0D Lead-Free (C4H14N2)2In2Br10 Single Crystal. Angew. Chem. Int. Ed. 2019, 58, 15435–15440. [Google Scholar] [CrossRef]
- Lin, R.; Guo, Q.; Zhu, Q.; Zhu, Y.; Zheng, W.; Huang, F. All-Inorganic CsCu2I3 Single Crystal with High-PLQY (≈15.7%) Intrinsic White-Light Emission via Strongly Localized 1D Excitonic Recombination. Adv. Mater. 2019, 31, 1905079. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, S.; Guo, Y.; Zhi, R.; Wang, X.; Ge, F.; Tian, Y.; Wang, J.; Zou, B. Highly Efficient Self-Trapped Exciton Emission of a (MA) 4Cu2Br6 Single Crystal. J. Phys. Chem. Lett. 2020, 11, 4703–4710. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S. The dawn of lead-free perovskite solar cell: Highly stable double perovskite Cs2AgBiBr6 film. Adv. Sci. 2018, 5, 1700759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, W.; Gao, F. Structural and functional diversity in lead-free halide perovskite materials. Adv. Mater. 2019, 31, 1900326. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wu, H.; Luo, J.; Deng, Z.; Ge, C.; Chen, C.; Jiang, X.; Yin, W.-J.; Niu, G.; Zhu, L.; et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nat. Photonics 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, G.; Zhou, C.; Chung, C.-C.; Hany, I. Optical and electrical properties of all-inorganic Cs2AgBiBr6 double perovskite single crystals. RSC Adv. 2019, 9, 23459–23464. [Google Scholar] [CrossRef] [Green Version]
- Steele, J.A.; Pan, W.; Martin, C.; Keshavarz, M.; Debroye, E.; Yuan, H.; Banerjee, S.; Fron, E.; Jonckheere, D.; Kim, C.W.; et al. Photophysical Pathways in Highly Sensitive Cs2 AgBiBr6 Double-Perovskite Single-Crystal X-Ray Detectors. Adv. Mater. 2018, 30, e1804450. [Google Scholar] [CrossRef]
- Zhang, Z.; Chung, C.-C.; Huang, Z.; Vetter, E.; Seyitliyev, D.; Sun, D.; Gundogdu, K.; Castellano, F.N.; Danilov, E.O.; Yang, G. Towards radiation detection using Cs2AgBiBr6 double perovskite single crystals. Mater. Lett. 2020, 269, 127667. [Google Scholar] [CrossRef]
- Keshavarz, M.; Debroye, E.; Ottesen, M.; Martin, C.; Zhang, H.; Fron, E.; Küchler, R.; Steele, J.A.; Bremholm, M.; Van de Vondel, J. Tuning the Structural and Optoelectronic Properties of Cs2AgBiBr6 Double-Perovskite Single Crystals through Alkali-Metal Substitution. Adv. Mater. 2020, 32, 2001878. [Google Scholar] [CrossRef]
- Yin, H.; Xian, Y.; Zhang, Y.; Chen, W.; Wen, X.; Rahman, N.U.; Long, Y.; Jia, B.; Fan, J.; Li, W. An Emerging Lead-Free Double-Perovskite Cs2AgFeCl6: In Single Crystal. Adv. Funct. Mater. 2020, 30, 2002225. [Google Scholar] [CrossRef]
- Luo, J.; Li, S.; Wu, H.; Zhou, Y.; Li, Y.; Liu, J.; Li, J.; Li, K.; Yi, F.; Niu, G. Cs2AgInCl6 double perovskite single crystals: Parity forbidden transitions and their application for sensitive and fast UV photodetectors. ACS Photonics 2018, 5, 398–405. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Liao, J.; Jiang, Y.; Kuang, D. Enhanced On–Off Ratio Photodetectors Based on Lead-Free Cs3Bi2I9 Single Crystal Thin Films. Adv. Funct. Mater. 2020, 30, 1909701. [Google Scholar] [CrossRef]
- Dang, Y.; Tong, G.; Song, W.; Liu, Z.; Qiu, L.; Ono, L.K.; Qi, Y. Interface engineering strategies towards Cs2AgBiBr6 single-crystalline photodetectors with good Ohmic contact behaviours. J. Mater. Chem. C 2020, 8, 276–284. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Zhang, S.; Li, J.; Wang, C.; Zhao, C.; Nie, P.; Dong, Y.; Zhang, X.; Zhao, S.; et al. Lead-Free Cs3Sb2Br9 Single Crystals for High Performance Narrowband Photodetector. Adv. Opt. Mater. 2020, 8, 2001072. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, Q.; Zhou, H.; Luo, P.; Nie, A.; Zhu, H.; Gan, L.; Zhuge, F.; Ma, Y.; Song, H.; et al. Submillimeter and lead-free Cs3Sb2Br9 perovskite nanoflakes: Inverse temperature crystallization growth and application for ultrasensitive photodetectors. Nanoscale Horiz. 2019, 4, 1372–1379. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, H.; Duan, C.; Yang, S.; Yang, Z.; Liu, Z.; Liu, S. Controlled n-Doping in Air-Stable CsPbI2Br Perovskite Solar Cells with a Record Efficiency of 16.79%. Adv. Funct. Mater. 2020, 30, 1909972. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Alsalloum, A.Y.; Turedi, B.; Zheng, X.; Mitra, S.; Zhumekenov, A.A.; Lee, K.J.; Maity, P.; Gereige, I.; AlSaggaf, A.; Roqan, I.S. Low-temperature crystallization enables 21.9% efficient single-crystal MAPbI3 inverted perovskite solar cells. ACS Energy Lett. 2020, 5, 657–662. [Google Scholar] [CrossRef]
- Chen, Z.; Turedi, B.; Alsalloum, A.Y.; Yang, C.; Zheng, X.; Gereige, I.; AlSaggaf, A.; Mohammed, O.F.; Bakr, O.M. Single-crystal MAPbI3 perovskite solar cells exceeding 21% power conversion efficiency. ACS Energy Lett. 2019, 4, 1258–1259. [Google Scholar] [CrossRef] [Green Version]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, S.; Cao, B.; Tao, X.; Chen, Z. Single Crystal Perovskite Solar Cells: Development and Perspectives. Adv. Funct. Mater. 2019, 30, 1905021. [Google Scholar] [CrossRef]
- He, L.; Gu, H.; Liu, X.; Li, P.; Dang, Y.; Liang, C.; Ono, L.K.; Qi, Y.; Tao, X. Efficient anti-solvent-free spin-coated and printed Sn-perovskite solar cells with crystal-based precursor solutions. Matter 2020, 2, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zuo, W.-W.; Yang, Y.-G.; Aldamasy, M.H.; Wang, Q.; Cruz, S.H.T.; Feng, S.-L.; Saliba, M.; Wang, Z.-K.; Abate, A. Tin halide perovskite films made of highly oriented 2D crystals enable more efficient and stable lead-free perovskite solar cells. ACS Energy Lett. 2020, 5, 1923–1929. [Google Scholar] [CrossRef]
- Shao, S.; Liu, J.; Portale, G.; Fang, H.; Blake, G.R.; ten Brink, G.H.; Koster, L.J.A.; Loi, M.A. Highly reproducible Sn-based hybrid perovskite solar cells with 9% efficiency. Adv. Energy Mater. 2018, 8, 1702019. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; He, X.; Wang, Y.; Meng, X.; Noda, T.; Yang, X.; Han, L. Efficient and stable tin-based perovskite solar cells by introducing π-conjugated Lewis base. Sci. China Chem. 2020, 63, 107–115. [Google Scholar] [CrossRef]
- Meng, X.; Wu, T.; Liu, X.; He, X.; Noda, T.; Wang, Y.; Segawa, H.; Han, L. Highly Reproducible and Efficient FASnI 3 Perovskite Solar Cells Fabricated with Volatilizable Reducing Solvent. J. Phys. Chem. Lett. 2020, 11, 2965–2971. [Google Scholar] [CrossRef]
- Jokar, E.; Chien, C.-H.; Fathi, A.; Rameez, M.; Chang, Y.-H.; Diau, E.W.-G. Slow surface passivation and crystal relaxation with additives to improve device performance and durability for tin-based perovskite solar cells. Energy Environ. Sci. 2018, 11, 2353–2362. [Google Scholar] [CrossRef]
- Wang, C.; Gu, F.; Zhao, Z.; Rao, H.; Qiu, Y.; Cai, Z.; Zhan, G.; Li, X.; Sun, B.; Yu, X.; et al. Self-Repairing Tin-Based Perovskite Solar Cells with a Breakthrough Efficiency Over 11%. Adv. Mater. 2020, 32, 1907623. [Google Scholar] [CrossRef]
- Zhao, B.; Abdi-Jalebi, M.; Tabachnyk, M.; Glass, H.; Kamboj, V.S.; Nie, W.; Pearson, A.J.; Puttisong, Y.; Gödel, K.C.; Beere, H.E.; et al. High Open-Circuit Voltages in Tin-Rich Low-Bandgap Perovskite-Based Planar Heterojunction Photovoltaics. Adv. Mater. 2017, 29, 1604744. [Google Scholar] [CrossRef]
- Sabba, D.; Mulmudi, H.K.; Prabhakar, R.R.; Krishnamoorthy, T.; Baikie, T.; Boix, P.P.; Mhaisalkar, S.; Mathews, N. Impact of Anionic Br–Substitution on Open Circuit Voltage in Lead Free Perovskite (CsSnI3-x Br x) Solar Cells. J. Phys. Chem. C 2015, 119, 1763–1767. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; He, T.; Li, S.; Wang, H.; Chen, Y.; Yuan, M.; Chen, J. Orientation Regulation of Tin-Based Reduced-Dimensional Perovskites for Highly Efficient and Stable Photovoltaics. Adv. Funct. Mater. 2019, 29, 1807696. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Wei, Q.; Li, H.; Shang, Y.; Zhou, W.; Wang, C.; Cheng, P.; Chen, Q.; Chen, L.; et al. Ultra-high open-circuit voltage of tin perovskite solar cells via an electron transporting layer design. Nat. Commun. 2020, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, T.; Xu, Z.; Liu, X.; Huang, X.; Han, S.; Liu, Y.; Li, M.; Luo, J.; Sun, Z. Dimensional Reduction of Cs2AgBiBr6: A 2D Hybrid Double Perovskite with Strong Polarization Sensitivity. Angew. Chem. Int. Ed. 2020, 59, 3429–3433. [Google Scholar] [CrossRef]
- Shi, C.; Ye, L.; Gong, Z.-X.; Ma, J.-J.; Wang, Q.-W.; Jiang, J.-Y.; Hua, M.-M.; Wang, C.-F.; Yu, H.; Zhang, Y.; et al. Two-Dimensional Organic–Inorganic Hybrid Rare-Earth Double Perovskite Ferroelectrics. J. Am. Chem. Soc. 2020, 142, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, X.; Han, S.; Liu, Y.; Xu, Z.; Hong, M.; Luo, J.; Sun, Z. Room-Temperature Ferroelectric Material Composed of a Two-Dimensional Metal Halide Double Perovskite for X-ray Detection. Angew. Chem. Int. Ed. 2020, 59, 13879–13884. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Li, M.; Cui, Y.; Song, X.; Wang, Q.; Jiang, J.; Hua, M.; Xu, Q.; Zhao, K.; et al. Centimeter-Sized Single Crystals of Two-Dimensional Hybrid Iodide Double Perovskite (4,4-Difluoropiperidinium) 4AgBiI8 for High-Temperature Ferroelectricity and Efficient X-Ray Detection. Adv. Funct. Mater. 2021, 31, 2009457. [Google Scholar] [CrossRef]
- Yakunin, S.; Dirin, D.N.; Shynkarenko, Y.; Morad, V.; Cherniukh, I.; Nazarenko, O.; Kreil, D.; Nauser, T.; Kovalenko, M.V. Detection of gamma photons using solution-grown single crystals of hybrid lead halide perovskites. Nat. Photonics 2016, 10, 585–589. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Xu, Q.; Wei, H.; Fang, Y.; Wang, Q.; Deng, Y.; Li, T.; Gruverman, A.; Cao, L.; et al. Monolithic integration of hybrid perovskite single crystals with heterogenous substrate for highly sensitive X-ray imaging. Nat. Photonics 2017, 11, 315–321. [Google Scholar] [CrossRef]
- Heiss, W.; Brabec, C. Perovskites target X-ray detection. Nat. Photonics 2016, 10, 288–289. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yang, Z.; Cui, J.; Wu, H.; Ren, X.; Zhao, K.; Feng, J.; Tang, J.; Xu, Z.; et al. Large Lead-Free Perovskite Single Crystal for High-Performance Coplanar X-Ray Imaging Applications. Adv. Opt. Mater. 2020, 8, 2000814. [Google Scholar] [CrossRef]
- Yin, L.; Wu, H.; Pan, W.; Yang, B.; Li, P.; Luo, J.; Niu, G.; Tang, J. Controlled cooling for synthesis of Cs2AgBiBr6 single crystals and its application for X-ray detection. Adv. Opt. Mater. 2019, 7, 1900491. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Yang, Z.; Zhang, Y.; Cui, J.; He, Y.; Ye, H.; Zhao, K.; Sun, H.; Lu, R. Inch-size 0D-structured lead-free perovskite single crystals for highly sensitive stable X-ray imaging. Matter 2020, 3, 180–196. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Li, Y.; Liu, X.; Yang, T.; Ji, C.; Han, S.; Xu, Y.; Luo, J.; Sun, Z. Exploring Lead-Free Hybrid Double Perovskite Crystals of (BA) 2CsAgBiBr7 with Large Mobility-Lifetime Product toward X-Ray Detection. Angew. Chem. Int. Ed. 2019, 58, 15757–15761. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, Z.; Ye, H.; Yang, Z.; You, J.; Liu, M.; He, Y.; Kanatzidis, M.G.; Liu, S. Nucleation-controlled growth of superior lead-free perovskite Cs3Bi2I9 single-crystals for high-performance X-ray detection. Nat. Commun. 2020, 11, 2304. [Google Scholar] [CrossRef]
- Tao, K.; Li, Y.; Ji, C.; Liu, X.; Wu, Z.; Han, S.; Sun, Z.; Luo, J. A Lead-Free Hybrid Iodide with Quantitative Response to X-ray Radiation. Chem. Mater. 2019, 31, 5927–5932. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, X.; Yang, Y.; Yang, S.; Zhao, W.; Wumaier, T.; Wei, D.; Deng, W.; Han, K. Air-Stable, Lead-Free Zero-Dimensional Mixed Bismuth-Antimony Perovskite Single Crystals with Ultra-broadband Emission. Angew. Chem. Int. Ed. Engl. 2019, 58, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, G.; Li, Y.; Wang, L.; Zhou, T.; Lin, Z.; Xie, R.J. Realizing Tunable White Light Emission in Lead-Free Indium(III) Bromine Hybrid Single Crystals through Antimony(III) Cation Doping. J. Phys. Chem. Lett. 2020, 11, 10164–10172. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, Y.; Jiang, X.; Molokeev, M.S.; Lin, Z.; Xia, Z. Sb3+ Dopant and Halogen Substitution Triggered Highly Efficient and Tunable Emission in Lead-Free Metal Halide Single Crystals. Chem. Mater. 2020, 32, 5327–5334. [Google Scholar] [CrossRef]


| Ion | Perovskite | Bandgap (eV) | Crystal System | Dimension | Synthesis | Ref. |
|---|---|---|---|---|---|---|
| Sn2+ | CsSnI3 | 1.31 | Orthorhombic | 3D | Bridgeman Method | [26] |
| Sn2+ | α-FASnI3 | N/A | Cubic | 3D | N/A | [27] |
| Sn2+ | β-FASnI3 | N/A | Tetragonal | 3D | N/A | [27] |
| Sn2+ | γ-FASnI3 | N/A | Tetragonal | 3D | N/A | [27] |
| Sn2+ | MASnI3 | 1.21 | Cubic | 3D | Cooling-induced crystallization method | [28] |
| Sn2+ | PEA2SnBr4 | 2.6 | Monoclinic | 2D | Cooling-induced crystallization method | [29] |
| Bi3+ | Rb3Bi2I9 | 2.1 | Monoclinic | 2D | Bridgeman Method | [30] |
| Bi3+ | Cs3Bi2I9 | 1.9/2.06 | Hexagonal | 0D | Bridgeman Method | [30,31] |
| Sb3+ | Rb3Sb2I9 | 2.03 | Monoclinic | 2D | Bridgeman Method | [31] |
| Sb3+ | Cs3Sb2I9 | 1.89 | Hexagonal | 2D | Bridgeman Method | [31,32] |
| N/A | Cs2AgBiBr6 | 2.1 | Cubic | 3D | Inverse temperature crystallization method | [40] |
| 2.25 | Cooling-induced crystallization method | [42] | ||||
| N/A | Cs2AgInCl6 | 3.2 | Cubic | 3D | Cooling-induced crystallization method | [46] |
| LEPSC | Responsivity | Detectivity (Jones) | ON-OFF Ratio | Ref. |
|---|---|---|---|---|
| Cs2AgInCl6 | 0.013 A W−1 | 9.60 × 1011 | NA | [46] |
| Cs3Bi2I9 | 7.2 × 10−3 A W−1 | 1.0 × 1011 | NA | [47] |
| Cs2AgBiBr6 | 0.92 A W−1 | 2.66 × 109 | 153 | [48] |
| Cs2AgBiBr6 | 0.9 mA W−1 | 1.38 × 109 | 42 | [48] |
| Cs3Bi2I9 | 2.29 A W−1 | 3.77 × 1012 | NA | [49] |
| LEPSCs | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
| FASnI3 | 0.63 | 21.60 | 74.7 | 10.17 | [62] |
| FASnI3 | 0.628 | 22.23 | 74.2 | 10.37 | [63] |
| FASnI3+1%EDAI2 | 0.58 | 21.3 | 72 | 8.9 | [64] |
| FASnI3+5%PHCl | 0.76 | 23.5 | 64 | 11.4 | [65] |
| CsSnI3 | 0.86 | 23.2 | 65 | 12.96 | [66] |
| (FA)0.75(MA)0.25SnI3+10%SnF2 | 0.61 | 21.2 | 62.7 | 8.12 | [67] |
| AVA2FAn−1SnnI3n+1 | 0.61 | 21.0 | 68 | 8.71 | [68] |
| PEAxFA1−xSnI3+NH4SC | 0.94 | 17.4 | 75 | 12.4 | [69] |
| LEPSCs | μτ Product (cm2V−1) | Sensitivity (μC·Gyair−1·cm−2) | Detection limit (nGyairs−1) | Ref. |
|---|---|---|---|---|
| Cs2AgBiBr6 | 6.3 × 10−3 | 316.8 | 59.7 | [40] |
| Cs2AgBiBr6 | 5.95 × 10−3 | 1974 | 226.2 | [78] |
| MA3Bi2I9 | NA | 1947 | 83 | [79] |
| Cs3Bi2I9 | 7.97 × 10−4 | 1652.3 | 130 | [81] |
| (BA)2CsAgBiBr7 | 1.21 × 10−3 | 4.2 | NA | [80] |
| (H2MDAP)BiI5 | NA | 1.0 | NA | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, Y.; Ge, C.; Tang, B.; Lin, H.; Zhang, X.; Huang, Y.; Zhu, Q.; Hu, H. Lead-Free Perovskite Single Crystals: A Brief Review. Crystals 2021, 11, 1329. https://doi.org/10.3390/cryst11111329
Zhou X, Wang Y, Ge C, Tang B, Lin H, Zhang X, Huang Y, Zhu Q, Hu H. Lead-Free Perovskite Single Crystals: A Brief Review. Crystals. 2021; 11(11):1329. https://doi.org/10.3390/cryst11111329
Chicago/Turabian StyleZhou, Xianfang, Yansong Wang, Chuangye Ge, Bin Tang, Haoran Lin, Xintao Zhang, Yun Huang, Quanyao Zhu, and Hanlin Hu. 2021. "Lead-Free Perovskite Single Crystals: A Brief Review" Crystals 11, no. 11: 1329. https://doi.org/10.3390/cryst11111329