Synthesis and Structures of TiIII and TiIV Complexes Supported by a Bulky Guanidinate Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Syntheses
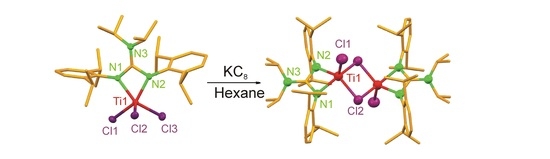
3. Results
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edelmann, F.T.; Hill, A.F.; Fink, M.J. Recent progress in the chemistry of metal amidinates and guanidinates: Syntheses, catalysis and materials. Adv. Organometal. Chem. 2013, 61, 55–374. [Google Scholar] [CrossRef]
- Edelmann, F.T. Advances in the coordination chemistry of amidinate and guanidinate ligands. Adv. Organometal. Chem. 2008, 57, 183–352. [Google Scholar] [CrossRef]
- Barker, J.; Kilner, M. The coordination chemistry of the amidine ligand. Coord. Chem. Rev. 1994, 133, 219–300. [Google Scholar] [CrossRef]
- Jones, C. Bulky guanidinates for the stabilization of low oxidation state metallacycles. Coord. Chem. Rev. 2010, 254, 1273–1289. [Google Scholar] [CrossRef]
- Bailey, P.J.; Pace, S. The coordination chemistry of guanidines and guanidinates. Coord. Chem. Rev. 2001, 214, 91–141. [Google Scholar] [CrossRef]
- Bourget-Merle, L.; Lappert, M.F.; Severn, J.R. The chemistry of β-diketiminatometal complexes. Chem. Rev. 2002, 120, 3031–3065. [Google Scholar] [CrossRef]
- Tsai, Y.-C. The chemistry of univalent metal β-diketiminates. Coord. Chem. Rev. 2012, 256, 722–758. [Google Scholar] [CrossRef]
- Chen, C.; Bellows, S.M.; Holland, P. Tuning steric and electronic effects in transition-metal β–diketiminate complexes. Dalton Trans. 2015, 44, 16654–16670. [Google Scholar] [CrossRef] [Green Version]
- Noor, A. Coordination chemistry of bulky aminopryridinates with main group and transition metals. Top. Curr. Chem. 2021, 379, 6. [Google Scholar] [CrossRef]
- Kempe, R. The strained η2-NAmido−NPyridine coordination of aminopyridinato ligands. Eur. J. Inorg. Chem. 2003, 791–803. [Google Scholar] [CrossRef]
- Deeken, S.; Motz, G.; Kempe, R. How common are true aminopyridinato complexes? Z. Anorg. Allg. Chem. 2007, 633, 320–325. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Wang, P.-Y.; Lin, K.-M.; Chen, S.-A.; Chen, J.-M. Synthesis and reactions of β-diketiminato divanadium(i) inverted-sandwich complexes. Chem. Commun. 2008, 205–207. [Google Scholar] [CrossRef]
- Wagner, F.R.; Noor, A.; Kempe, R. Ultrashort metal–metal distances and extreme bond orders. Nat. Chem. 2009, 1, 322–325. [Google Scholar] [CrossRef]
- Noor, A.; Kempe, R. The shortest metal-metal bond. Chem. Rec. 2010, 10, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Kempe, R. M5M—Key compounds of the research field metal–metal quintuple bonding. Inorg. Chim. Acta 2014, 424, 75–82. [Google Scholar] [CrossRef]
- Fohlmeister, L.; Liu, S.; Schulten, C.; Moubaraki, B.; Stasch, A.; Cashion, J.D.; Murray, K.S.; Gagliardi, L.; Jones, C. Low-Coordinate Iron(I) and Manganese(I) Dimers: Kinetic Stabilization of an Exceptionally Short Fe-Fe Multiple Bond. Angew. Chem. Int. Ed. 2012, 51, 8294–8298. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Bauer, T.; Todorova, T.K.; Weber, B.; Gagliardi, L.; Kempe, R. The ligand-based quintuple bond-shortening concept and some of its limitations. Chem. Eur. J. 2013, 19, 9825–9832. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Glatz, G.; Müller, R.; Kaupp, M.; Demeshko, S.; Kempe, R. Metal–metal distances at the limit: Cr–Cr 1.73 Å—The importance of the ligand and its fine tuning. Z. Anorg. Allg. Chem. 2009, 635, 119–1152. [Google Scholar] [CrossRef]
- Rosenthal, U. Recent synthetic and catalytic applications of group 4 metallocene bis(trimethylsilyl)acetylene complexes. Eur. J. Inorg. 2019, 895–919. [Google Scholar] [CrossRef]
- Noor, A.; Kempe, R. Acetylenetitanium complex stabilized by aminopyridinato ligands. Eur. J. Inorg. 2008, 2377–2381. [Google Scholar] [CrossRef]
- Nikiforov, G.B.; Crewdson, P.; Gambarotta, S.; Korobkov, I.; Budzelaar, P.H.M. Reduction of titanium supported by a σ-/π-bonded tripyrrole ligand: Ligand C−N bond cleavage and coordination of olefin and arene with an inverse sandwich structure. Organometallics 2007, 26, 48–55. [Google Scholar] [CrossRef]
- Benzing, E.; Kornicker, W. Dialkylamido-titan(IV)-chloride und –alkoholate. Chem. Ber. 1961, 94, 2263–2267. [Google Scholar] [CrossRef]
- Green, S.P.; Jones, C.; Stasch, A. Stable magnesium(I) compounds with Mg-Mg bonds. Science 2007, 318, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR 97: A new tool for crystal determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sec. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGX Suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Pillai, S.K.T.; Kretschmer, W.P.; Trebbin, M.; Foerster, S.; Kempe, R. Tailored nanostructuring of end-group-functionalized high-density polyethylene synthesized by an efficient catalytic version of Zieglers “aufbaureaktion”. Chem. Eur. J. 2012, 18, 13974–13978. [Google Scholar] [CrossRef]
- Obenauf, J.; Kretschmer, W.P.; Kempe, R. Efficient synthesis of aluminium-terminated polyethylene by means of irreversible coordinative chain-transfer polymerisation using a guanidinatotitanium catalyst. Eur. J. Inorg. Chem. 2014, 2014, 1446–1453. [Google Scholar] [CrossRef]
- Wasslen, Y.A.; Tois, E.; Haukka, S.; Kreisel, K.A.; Yap, G.P.A.; Halls, M.D.; Barry, S.T. A family of heteroleptic titanium guanidinates: Synthesis, thermolysis, and surface reactivity. Inorg. Chem. 2010, 49, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Calderon, J.R.; Murillo, J.; Gomez-Torres, A.; Saucedo, C.; Jordan, A.; Metta-Magana, A.J.; Pink, M.; Fortier, S. Redox character and small molecule reactivity of a masked titanium(II) synthon. Organometallics 2020, 39, 295–311. [Google Scholar] [CrossRef] [Green Version]
- Coles, M.P.; Hitchcock, P.B. Exploration of the Suitability of Bicyclic Guanidinates as Ligands in Catalytic Chemistry Mediated by Titanium. Organometallics 2003, 22, 5201–5211. [Google Scholar] [CrossRef]
- Minhas, R.; Duchateau, R.; Gambarrota, S.; Bensimon, C. Linear trimeric, dimeric, and monomeric titanium(III) aryloxides. Inorg. Chem. 1992, 31, 4933–4938. [Google Scholar] [CrossRef]
- Matsuo, T.; Kawaguchi, H.; Sakai, M. Synthesis and structures of Ti(III) and Ti(IV) complexes supported by a tridentate aryloxide ligand. J. Chem. Soc. Dalton Trans. 2002, 2536–2540. [Google Scholar] [CrossRef]





| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C31H48Cl3N3Ti | C68H110Cl4N6Ti2 |
| Formula weight | 616.97 | 1249.22 |
| crystal system | orthorhombic | monoclinic |
| space group | Pna2(1) | C2/c |
| a [Å] | 19.4050(9) | 36.6350(15) |
| b [Å] | 10.5550(4) | 11.2480(8) |
| c [Å] | 16.1720(7) | 19.9120(15) |
| α [deg] | 90.00 | 90.00 |
| β [deg] | 90.00 | 120.746(6) |
| γ [deg] | 90.00 | 90.00 |
| V, [Å3] | 3312.3(2) | 7051.8(8) |
| crystal size, [mm3] | 0.43 × 0.32 × 0.25 | 0.38 × 0.36 × 0.33 |
| ρcalcd, [g cm−3] | 1.237 | 1.177 |
| µ, [mm−1] (Mo Kα) | 0.524 | 0.420 |
| T, [K] | 133(2) | 133(2) |
| 2θ range, [deg] | 2.52–52.06 | 2.59–45.42 |
| no. of reflections unique | 6232 | 6661 |
| no. of reflections obs. [I > 2σ (I)] | 5081 | 2099 |
| no. of parameters | 343 | 374 |
| wR2 (all data) | 0.0687 | 0.1999 |
| R value [I > 2σ (I)] | 0.0351 | 0.0747 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A. Synthesis and Structures of TiIII and TiIV Complexes Supported by a Bulky Guanidinate Ligand. Crystals 2021, 11, 886. https://doi.org/10.3390/cryst11080886
Noor A. Synthesis and Structures of TiIII and TiIV Complexes Supported by a Bulky Guanidinate Ligand. Crystals. 2021; 11(8):886. https://doi.org/10.3390/cryst11080886
Chicago/Turabian StyleNoor, Awal. 2021. "Synthesis and Structures of TiIII and TiIV Complexes Supported by a Bulky Guanidinate Ligand" Crystals 11, no. 8: 886. https://doi.org/10.3390/cryst11080886