Synthesis and Structural Characterization of Isostructural 4-(4-Aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of 2
2.3. Synthesis of 3
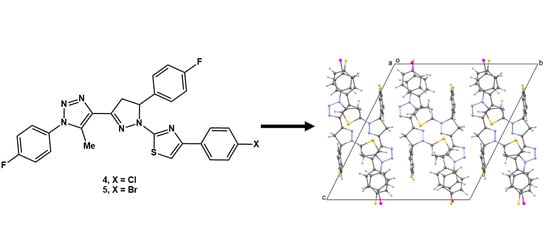
2.4. Synthesis of 4 and 5
2.5. X-ray Crystal Structure
3. Results and Discussion
3.1. Synthesis of Compounds 4 and 5
3.2. Crystal Structures of 4 and 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Hashem, A.A. Synthesis of new furothiazolo pyrimido quinazolinones from visnagenone or khellinone and antimicrobial activity. Molecules 2018, 23, 2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jampilek, J. Heterocycles in medicinal chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Gondru, R.; Kanugala, S.; Raj, S.; Kumar, C.G.; Pasupuleti, M.; Banothu, J.; Bavantula, R. 1,2,3-Triazole-thiazole hybrids: Synthesis, in vitro antimicrobial activity and antibiofilm studies. Bioorg. Med. Chem. Lett. 2020, 33, 127746. [Google Scholar] [CrossRef] [PubMed]
- El Malah, T.; Nour, H.F.; Satti, A.A.E.; Hemdan, B.A.; El-Sayed, W.A. Design, synthesis, and antimicrobial activities of 1,2,3-triazole glycoside clickamers. Molecules 2020, 25, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellouz, M.; Sebbar, N.K.; Fichtali, I.; Ouzidan, Y.; Mennane, Z.; Charof, R.; Mague, J.T.; Urrutigoïty, M.; Essassi, E.M. Synthesis and antibacterial activity of new 1,2,3-triazolylmethyl-2H-1,4-benzothiazin-3(4H)-one derivatives. Chem. Cent. J. 2018, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; Masłyk, M.; Estévez-Braun, A. Synthesis and antimicrobial activity of 4-substituted 1,2,3-triazole-coumarin derivatives. Molecules 2018, 23, 199. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.D.C.; De Souza, M.C.B.V.; Frugulhetti, I.I.P.; Castro, H.C.; Souza, S.L.D.O.; De Souza, T.M.L.; Rodrigues, D.Q.; Souza, A.M.T.; Abreu, P.A.; Passamani, F.; et al. Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009, 44, 373–383. [Google Scholar] [CrossRef]
- Doiron, J.; Soultan, A.H.; Richard, R.; Touré, M.M.; Picot, N.; Richard, R.; Cuperlović-Culf, M.; Robichaud, G.A.; Touaibia, M. Synthesis and structure activity relationship of 1- and 2-substituted-1,2,3-triazole letrozole-based analogues as aromatase inhibitors. Eur. J. Med. Chem. 2011, 46, 4010–4024. [Google Scholar] [CrossRef]
- Sert, Y.; El-Hiti, G.A.; Gökce, H.; Ucun, F.; Abdel-Wahab, B.F.; Kariuki, B.M. DFT, molecular docking and experimental FT-IR, laser-Raman, NMR and UV investigations on a potential anticancer agent containing triazole ring system. J. Mol. Struct. 2020, 1211, 128077. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Abdel-Wahab, B.F.; Bekheit, M.S. Synthesis, molecular docking, and evaluation of novel bivalent pyrazolinyl-1,2,3-triazoles as potential VEGFR TK inhibitors and anti-cancer agents. Chem. Pap. 2018, 72, 2225–2237. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Khidre, R.E.; Awad, G.E.A. Design and synthesis of novel 6-(5-methyl-1H-1,2,3-triazol-4-yl)-5-[(2-(thiazol-2-yl)hydrazono)methyl]imidazo[2,1-b]thiazoles as antimicrobial agents. J. Heterocycl. Chem. 2017, 54, 489–494. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Alotaibi, M.H.; El-Hiti, G.A. Synthesis of new symmetrical N,N′-diacylhydrazines and 2-(1,2,3-triazol-4-yl)-1,3,4-oxadiazoles. Lett. Org. Chem. 2017, 14, 591–596. [Google Scholar] [CrossRef]
- Penthala, N.R.; Madhukuri, L.; Thakkar, S.; Madadi, N.R.; Lamture, G.; Eoff, R.L.; Crooks, P.A. Synthesis and anti-cancer screening of novel heterocyclic-(2H)-1,2,3-triazoles as potential anti-cancer agents. Med. Chem. Commun. 2015, 6, 1535–1543. [Google Scholar] [CrossRef] [Green Version]
- Stefely, J.A.; Palchaudhuri, R.; Miller, P.A.; Peterson, R.J.; Moraski, G.C.; Hergenrother, P.J.; Miller, M.J. N-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl) arylamide as a new scaffold that provides rapid access to antimicrotubule agents: Synthesis and evaluation of antiproliferative activity against select cancer cell lines. J. Med. Chem. 2010, 53, 3389–3395. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Huang, X.; Yao, H.; Jiang, J.; Wu, X.; Jiang, S.; Wang, Q.; Lu, T.; Xu, J. Discovery of potential anti-inflammatory drugs: Diaryl-1,2,4-triazoles bearing N-hydroxyurea moiety as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. Org. Biomol. Chem. 2014, 12, 2114–2127. [Google Scholar] [CrossRef] [Green Version]
- Huisgen, R.; Szeimies, G.; Möbius, L. 1.3-Dipolare cycloadditionen, XXXII. Kinetik der additionen organischer azide an CC-mehrfachbindungen. Chem. Ber. 1967, 100, 2494–2507. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Seus, N.; Gonçalves, L.C.; Deobald, A.M.; Savegnago, L.; Alves, D.; Paixão, M.W. Synthesis of arylselanyl-1H-1,2,3-triazole-4-carboxylates by organocatalytic cycloaddition of azidophenyl arylselenides with β-keto-esters. Tetrahedron 2012, 68, 10456–10463. [Google Scholar] [CrossRef]
- Schmieder, P.; Khne, R.; Rademann, J. Metal-free, regioselective triazole ligations that deliver locked cis peptide mimetics. Angew. Chem. Int. Ed. 2009, 48, 5042–5045. [Google Scholar] [CrossRef]
- Dey, S.; Pathak, T.A. General route to 1,5-disubstituted 1,2,3-triazoles with alkyl/alkyl, alkyl/aryl, aryl/aryl combinations: A metal-free, regioselective, one-pot three component approach. RSC Adv. 2014, 4, 9275–9278. [Google Scholar] [CrossRef]
- Cai, Z.-J.; Lu, X.-M.; Zi, Y.; Yang, C.; Shen, L.-J.; Li, J.; Wang, S.-Y.; Ji, S.-J. I2/TBPB mediated oxidative reaction of N-tosylhydrazones with anilines: Practical construction of 1,4-disubstituted 1,2,3-triazoles under metal-free and azide free conditions. Org. Lett. 2014, 16, 5108–5111. [Google Scholar] [CrossRef]
- Shaaban, M.R.; Mayhoub, A.S.; Farag, A.M. Recent advances in the therapeutic applications of pyrazolines. Expert. Opin. Ther. Pat. 2012, 22, 253–291. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M.Z. Unveiling a versatile heterocycle: Pyrazoline—A review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef] [Green Version]
- Tok, F.; Abas, B.I.; Çevik, O. Koçyiğit-Kaymakçıoğlu, B. Design, synthesis and biological evaluation of some new 2-Pyrazoline derivatives as potential anticancer agents. Bioorg. Chem. 2020, 102, 104063. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Jain, P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab. J. Chem. 2014, 7, 553–596. [Google Scholar] [CrossRef] [Green Version]
- Lellek, V.; Chen, C.-Y.; Yang, W.; Liu, J.; Ji, X.; Faessler, R. An efficient synthesis of substituted pyrazoles from one-pot reaction of ketones, aldehydes, and hydrazine monohydrochloride. Synlett 2018, 29, 1071–1075. [Google Scholar] [CrossRef] [Green Version]
- Alex, K.; Tillack, A.; Schwarz, N.; Beller, M. Zinc-catalyzed synthesis of pyrazolines and pyrazoles via hydrohydrazination. Org. Lett. 2008, 10, 2377–2379. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Sharma, P.K.; Amin, A.; Kumar, M. A review: Medicinally important nitrogen sulphur containing heterocycles. Open Med. Chem. J. 2021, 14, 49–64. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, X.; Wei, J.; Liu, J.; Song, S.; Wang, W.; Jiao, N. Cu-catalyzed aerobic oxidative sulfuration/annulation approach to thiazoles via multiple Csp 3-H bond cleavage. Org. Lett. 2018, 20, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, V.; Avellar, M.M.; Nery, A.C.S.; Gomes, R.B.; Vasconcelos, T.R.A.; de Souza, M.V.N. An eco-friendly, Hantzsch-based, solvent-free approach to 2-aminothiazoles and 2-aminoselenazoles. Synthesis 2016, 48, 437–440. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Alshammari, M.B.; El-Hiti, G.A. Synthesis of new 6-(1H-1,2,3-triazol-4-yl)-5-(1-(thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)imidazo[2,1-b]thiazoles. Indian J. Heterocycl. Chem. 2018, 28, 529–533. [Google Scholar]
- Abdel-Wahab, B.F.; Khidre, R.E.; Mohamed, H.A.; El-Hiti, G.A. A simple process for the synthesis of novel pyrazolyltriazole and dihydropyrazolylthiazole derivatives as antimicrobial agents. Arab. J. Sci. Eng. 2017, 42, 2441–2448. [Google Scholar] [CrossRef]
- Ghabbour, H.A.; Abdel-Wahab, B.F.; Alamri, M.; Al-Omar, M.A.; El-Hiti, G.A. Crystal structure of 2-(5-(4-fluorophenyl)-3-p-tolyl-4,5-dihydro-1H-pyrazol-1-yl)-4-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)thiazole, C29H25FN6S. Z. Kristallogr. New Cryst. Struct. 2017, 232, 21–23. [Google Scholar] [CrossRef] [Green Version]
- El-Hiti, G.A.; Mohamed, H.A.; Abdel-Wahab, B.F.; Alotaibi, M.H.; Hegazy, A.S.; Kariuki, B.M. 4-(4-Bromophenyl)-2-(3-(4-chlorophenyl)-5-{3-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl]-1-phenyl-1H-pyrazol-4-yl}-4,5-dihydro-1H-pyrazol-1-yl)thiazole. IUCrData 2018, 3, x180036. [Google Scholar] [CrossRef] [Green Version]
- Braga, D.; Desiraju, G.R.; Miller, J.S.; Orpend, A.G.; Price, S.L. Innovation in crystal engineering. CrystEngComm 2002, 4, 500–509. [Google Scholar] [CrossRef]
- Ebenezer, S.; Muthiah, P.T.; Butcher, R.J. Design of a series of isostructural co-crystals with aminopyrimidines: Isostructurality through chloro/methyl exchange and studies on supramolecular architectures. Cryst. Growth Des. 2011, 11, 3579–3592. [Google Scholar] [CrossRef]
- Chia, T.S.; Quah, C.K. Temperature-induced order-disorder structural phase transitions of two-dimensional isostructural hexamethylenetetramine co-crystals. Acta Crystallogr. Sect. B 2017, 73, 879–890. [Google Scholar] [CrossRef]
- Panicker, L. Thermal, spectroscopic and structural characterization of isostructural phase transition in 4-hydroxybenzaldehyde. Phase Transit. 2018, 91, 530–536. [Google Scholar] [CrossRef]
- Galcera, J.; Friščić, T.; Hejczyk, K.E.; Fábián, L.; Clarke, S.M.; Day, G.M.; Molinsa, E.; Jones, W. Isostructural organic binary-host frameworks with tuneable and diversely decorated inclusion cavities. CrystEngComm 2012, 14, 7898–7906. [Google Scholar] [CrossRef]
- Reddy, C.M.; Kirchner, M.T.; Gundakaram, R.C.; Padmanabhan, K.A.; Desiraju, G.R. Isostructurality, polymorphism and mechanical properties of some hexahalogenated benzenes: The nature of halogen···halogen interactions. Chem. Eur. J. 2006, 12, 2222–2234. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Saha, B.K. The effect of temperature on interhalogen interactions in a series of isostructural organic systems. New J. Chem. 2014, 38, 897–901. [Google Scholar] [CrossRef]
- Cincic, D.; Friscic, T.; Jones, W. A cocrystallisation-based strategy to construct isostructural solids. New J. Chem. 2008, 32, 1776–1781. [Google Scholar] [CrossRef]
- Kanao, S.; Kashino, S.; Haisa, M. Topochemical studies. XIII. Structures of 3-bromocinnamic acid and 3-chlorocinnamic acid. Acta Cryst. 1990, C46, 2436–2438. [Google Scholar] [CrossRef]
- Gougoutas, J.Z.; Lessinger, L. Solid state chemistry of organic polyvalent iodine compounds. IV. Topotactic transformations of 2-iodo-3′-chlorodibenzoyl peroxide and the crystal structure of m-chlorobenzoic acid. J. Solid State Chem. 1975, 12, 51–62. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Hibbs, D.E.; Ramachandran, V.N. 3-Chlorobenzoic acid. Private communication to the Cambridge Structural Database. 2003; MCBZAC01. [Google Scholar]
- Abdel-Wahab, B.F.; Abdel-Latif, E.; Mohamed, H.A.; Awad, G.E.A. Design and synthesis of new 4-pyrazolin-3-yl-1,2,3-triazoles and 1,2,3-triazol-4-yl-pyrazolin-1-ylthiazoles as potential antimicrobial agents. Eur. J. Med. Chem. 2012, 52, 263–268. [Google Scholar] [CrossRef]
- Kamalraj, V.R.; Senthil, S.; Kannan, P. One-pot synthesis and the fluorescent behavior of 4-acetyl-5-methyl-1,2,3-triazole regioisomers. J. Mol. Struct. 2008, 892, 210–215. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- MacKenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.-J.; Cui, F.-H.; Gao, Z.-L.; Li, R.-S.; Shen, G.-L.; Dong, H.-S. An efficient synthesis of 5-aryl-4,5-dihydro-3-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)-1-(4-phenylthiazol-2-yl)pyrazoles. J. Heterocycl. Chem. 2011, 48, 1154–1160. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Ng, S.W.; Tiekink, E.R.T. 4-{1-[4-(4-Bromophenyl)-1,3-thiazol-2-yl]- 5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-3-yl}-5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1956–o1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Wahab, B.F.; Ng, S.W.; Tiekink, E.R.T. 4-[5-(4-Fluorophenyl)-1-(4-phenyl-1,3-thiazol-2-yl)-4,5-dihydro-1H-pyrazol- 3-yl]-5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole. Acta Crystallogr. Sect. E Struct. Rep. Online 2013, 69, o618. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| 4 | 5 | |
|---|---|---|
| Formula | C27H19ClF2N6S | C27H19BrF2N6S |
| Formula weight | 532.99 | 577.45 |
| Temperature/K | 293(2) | 293(2) |
| Wavelength/Å | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic |
| Space group | PĪ | PĪ |
| a/Å | 7.7344(6) | 7.7607(3) |
| b/Å | 18.2778(12) | 18.2950(11) |
| c/Å | 19.4909(12) | 19.5252(14) |
| α/° | 116.181(6) | 115.910(6) |
| β/° | 96.410(6) | 96.971(4) |
| γ/° | 92.091(6) | 92.567(4) |
| Volume/Å3 | 2445.9(3) | 2460.2(3) |
| Z | 4 | 4 |
| Density (calculated)/Mg m−3 | 1.447 | 1.559 |
| μ/mm−1 | 0.287 | 1.801 |
| F(000) | 1096 | 1168 |
| Crystal size/mm3 | 0.270 × 0.065 × 0.026 | 0.623 × 0.148 × 0.118 |
| Reflections collected | 23,275 | 23,703 |
| Independent reflections | 11,546 | 11,617 |
| R(int) | 0.0496 | 0.0448 |
| Data/parameters | 11,546/670 | 11,617/669 |
| Goodness-of-fit on F2 | 1.014 | 1.032 |
| R1 [I > 2σ(I)] | 0.0652 | 0.0669 |
| wR2 [I > 2σ (I)] | 0.1239 | 0.1722 |
| R1 (all data) | 0.1802 | 0.1364 |
| wR2 (all data) | 0.1666 | 0.2137 |
| Extinction coefficient | 0.0012(3) | n/a |
| Largest diff. peak and hole/e.Å−3 | 0.238 and −0.243 | 0.689 and −0.680 |
| Inter-Ring Twist Angle | 4(i) | 4(ii) | 5(i) | 5(ii) |
| A–B | 9.44 (11) | 13.38 (10) | 9.68 (13) | 13.36 (14) |
| B–C | 3.58 (14) | 5.30 (15) | 5.17 (17) | 5.03 (20) |
| C–D | 88.15 (1) | 84.53 (13) | 88.15 (16) | 84.17 (16) |
| C–E | 10.39 (15) | 10.78 (16) | 10.86 (18) | 10.53 (21) |
| E–F | 33.09 (9) | 32.59 (10) | 35.39 (11) | 31.86(13) |
| Centroid-centroid distance | 4 | 5 | ||
| d1 | 3.75(1) | 3.73(1) | ||
| d2 | 4.05(1) | 4.09(1) | ||
| d3 | 3.81(1) | 3.79(1) | ||
| d4 | 4.14(1) | 4.20(1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, B.M.; Abdel-Wahab, B.F.; El-Hiti, G.A. Synthesis and Structural Characterization of Isostructural 4-(4-Aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals 2021, 11, 795. https://doi.org/10.3390/cryst11070795
Kariuki BM, Abdel-Wahab BF, El-Hiti GA. Synthesis and Structural Characterization of Isostructural 4-(4-Aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals. 2021; 11(7):795. https://doi.org/10.3390/cryst11070795
Chicago/Turabian StyleKariuki, Benson M., Bakr F. Abdel-Wahab, and Gamal A. El-Hiti. 2021. "Synthesis and Structural Characterization of Isostructural 4-(4-Aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles" Crystals 11, no. 7: 795. https://doi.org/10.3390/cryst11070795