Low Temperature Transitional Aluminas: Structure Specifics and Related X-ray Diffraction Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Crystallographic Model of the Structure
2.2. Modeling Technique
3. Results and Discussion
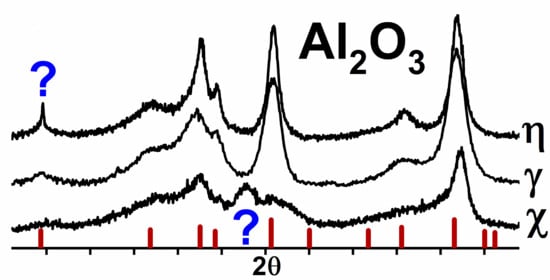
3.1. Nanostructure-Related Diffraction Effects by the Example of γ-Al2O3
3.2. Diffraction Feature of χ-Al2O3, Which Is Not Typical for the Spinel Structure
3.3. Nanostructure-Related Diffraction Features for η-Al2O3
4. Conclusions
- The observed diffraction features are related to the presence of coherent nanostructures in which the primary crystalline blocks are joined to each other with maintaining the mutual orientation and parallelism of the atomic planes. All the violations of the ideal crystal structure are associated with a slip in the joining planes.
- The possibilities of DSE for determining the various nanostructures inherent in all the low-temperature Al2O3 polymorphs are shown.
- It is shown that the observed diffraction differences between the analyzed Al2O3 polymorphs are primarily associated with the peculiarities of the nanostructure (the ways of mutual joining primary crystalline blocks). The atomic structure of individual Al2O3 crystalline blocks corresponds to a spinel-type structure.
- The primary crystalline blocks in particles of the low-temperature Al2O3 polymorphs (γ-, χ-, η-) are joined in a partially coherent way with preserved atomic order in the oxygen sublattice and planar defects disrupting the cationic sublattice.
- It is shown that the characteristic peak at 2θ~42.8° in XRD pattern of χ-Al2O3 is associated with the presence of fragments (domains) with a hexagonal close packing of oxygen atoms coherently joined along the (111) planes with domains with a cubic structure.
- Primary crystallites of η-Al2O3 have an anisotropic acicular shape due to their larger size in the preferred direction [111].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meephoka, C.; Chaisuk, C.; Samparnpiboon, P.; Praserthdam, P. Effect of phase composition between nano χ- and γ-Al2O3 on Pt/Al2O3 catalyst in CO oxidation. Catal. Commun. 2008, 9, 546–550. [Google Scholar] [CrossRef]
- Chaitree, W.; Jiemsirilers, S.; Mekasuwandumrong, O.; Jongsomjit, B.; Shotipruk, A.; Panpranot, J. Effect of nanocrystalline χ-Al2O3 structure on the catalytic behavior of Co/Al2O3 in CO hydrogenation. Catal. Today 2011, 164, 302–307. [Google Scholar] [CrossRef]
- Macleod, N.; Keel, J.M.; Lambert, R.M. The effects of catalyst aging under industrial conditions: Ethylene oxide conversion over Ag–Cs/α-Al2O3 catalysts. Catal. Lett. 2003, 86, 51–56. [Google Scholar] [CrossRef]
- Busca, G. Chapter Three—Structural, Surface, and Catalytic Properties of Aluminas. Adv. Catal. 2014, 57, 319–404. [Google Scholar]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 17, 3393–3403. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.-W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on γ-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef]
- Phung, T.K.; Lagazzo, A.; Crespo, M.Á.R.; Escribano, V.S.; Busca, G. A study of commercial transition aluminas and of their catalytic activity in the dehydration of ethanol. J. Catal. 2014, 311, 102–113. [Google Scholar] [CrossRef]
- Nazimov, D.A.; Klimov, O.V.; Trukhan, S.N.; Cherepanova, S.V.; Prosvirin, I.P.; Noskov, A.S. The Effect of Transition Alumina (γ-, η-, χ-Al2O3) on the Activity and Stability of Chromia/Alumina Catalysts. Part I: Model Catalysts and Aging Conditions. Energy Technol. 2019, 7, 1800735. [Google Scholar] [CrossRef]
- Kazakova, M.A.; Vatutina, Y.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Shuvaev, A.V.; Klimov, O.V.; Noskov, A.S.; Kazakov, M.O. Boosting hydrodesulfurization activity of CoMo/Al2O3 catalyst via selective graphitization of alumina surface. Microporous Mesoporous Mater. 2021, 317, 111008. [Google Scholar] [CrossRef]
- Busca, G. The surface of transitional aluminas: A critical review. Catal. Today 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Study of phase transformations during calcination of aluminum hydroxides by selected area electron diffraction. Acta Crystallogr. 1964, 17, 1312–1321. [Google Scholar] [CrossRef]
- Ushakov, V.A.; Moroz, E.M. Structure of low-temperature γ- and η-Al2O3. React. Kinet. Catal. Lett. 1984, 24, 113–118. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hunter, B.A.; Hart, R.D.; Hanna, J.V.; Byrne, L.T. Tetragonal Structure Model for Boehmite-Derived γ-Alumina. Phys. Rev. B 2003, 68, 144110. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Garcia-Garcia, F.J.; Häussermann, U. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Tsybulya, S.V.; Kryukova, G.N. Nanocrystalline transition aluminas: Nanostructure and features of X-ray powder diffraction patterns of low-temperature Al2O3 polymorphs. Phys. Rev. B 2008, 77, 024112. [Google Scholar] [CrossRef]
- Popescu, C.; Sans, J.A.; Errandonea, D.; Segura, A.; Villanueva, R.; Sapiña, F. Compressibility and Structural Stability of Nanocrystalline TiO2 Anatase Synthesized from Freeze-Dried Precursors. Inorg. Chem. 2014, 53, 11598–11603. [Google Scholar] [CrossRef]
- Daviau, K.; Lee, K.K.M. High-Pressure, High-Temperature Behavior of Silicon Carbide: A Review. Crystals 2018, 8, 217. [Google Scholar] [CrossRef] [Green Version]
- Pakharukova, V.P.; Yatsenko, D.A.; Gerasimov, E.Y.; Shalygin, A.S.; Martyanov, O.N.; Tsybulya, S.V. Coherent 3D nanostructure of γ-Al2O3: Simulation of whole X-ray powder diffraction pattern. J. Solid State Chem. 2017, 246, 284–292. [Google Scholar] [CrossRef]
- Rudolph, M.; Motylenko, M.; Rafaja, D. Structure model of γ-Al2O3 based on planar defects. IUCrJ 2019, 6, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.S.; Litvak, G.S.; Kryukova, G.N.; Tsybulya, S.V.; Paukshtis, E.A. Real structure of metastable forms of aluminum oxide. Kinet. Catal. 2000, 41, 122–126. [Google Scholar] [CrossRef]
- Kul’ko, E.V.; Ivanova, A.S.; Litvak, G.S.; Kryukova, G.N.; Tsybulya, S.V. Preparation and Microstructural and Textural Characterization of Single-Phase Aluminum Oxides. Kinet. Catal. 2004, 45, 714–721. [Google Scholar] [CrossRef]
- Wolverton, C.; Hass, K.C. Phase stability and structure of spinel-based transition aluminas. Phys. Rev. B 2000, 63, 024102. [Google Scholar] [CrossRef]
- Saalfeld, H.; Mehrota, B.B. Electron-diffraction study of aluminum oxides. Ber. Dtsch. Keram. Ges. 1965, 42, 161–166. [Google Scholar]
- Jayaram, V.; Levi, C.G. The structure of δ-alumina evolved from the melt and the γ → δ transformation. Acta Metall. 1989, 37, 569–578. [Google Scholar] [CrossRef]
- Wang, Y.G.; Bronsveld, P.M.; DeHosson, J.T.M. Ordering of Octahedral Vacancies in Transition Aluminas. J. Am. Ceram. Soc. 1998, 81, 1655. [Google Scholar] [CrossRef] [Green Version]
- Ruberto, C.; Yourdshahyan, Y.; Lundqvist, B.I. Surface properties of metastable alumina: A comparative study of κ- and α-Al2O3. Phys. Rev. B 2003, 67, 195412. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhou, R.S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Crystallogr. Sect. B 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Drits, V.A.; Tchoubar, C. X-ray Diffraction by Disordered Lamellar Structures; Springer: Berlin/Heidelberg, Germany, 1990; p. 371. [Google Scholar]
- Cherepanova, S.V.; Tsybulya, S.V. Simulation of X-ray powder diffraction patterns for one-dimensionally disordered crystals. Mat. Sci. Forum 2004, 443–444, 87–90. [Google Scholar] [CrossRef]
- Debye, P. Zerstreung von Röntgenstrahlen. Ann. Physik. 1915, 351, 809–823. [Google Scholar] [CrossRef] [Green Version]
- Tsybulya, S.V.; Yatsenko, D.A. X-ray diffraction analysis of ultradisperse systems: The Debye formula. J. Struct. Chem. 2012, 53, S150–S165. [Google Scholar] [CrossRef]
- Gelisio, L.; Scardi, P. 100 years of Debye’s scattering equation. Acta Crystallogr. Sect. A 2016, 72, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatsenko, D.; Tsybulya, S. DIANNA (Diffraction Analysis of Nanopowders)—A software for structural analysis of nanosized powders. Z. Kristallogr. Cryst. Mater. 2018, 233, 61–66. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nach. Gesell. Wissen. Göttingen 1918, 2, 98–100. [Google Scholar] [CrossRef]
- Shirai, T.; Watanabe, H.; Fuji, M.; Takahashi, M. Structural Properties and Surface Characteristics on Aluminum Oxide Powders. 2009, 9, 23–31. Annu. Rep. Ceram. Res. Lab. Nagoya Inst. Technol. 2009, 9, 23–31. [Google Scholar]








| Temperature, °C | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 | 1000 | 1100 | 1200 | 1300 |
| Bayerite Al(OH)3 | η | θ | α-Al2O3 | ||||||||||
| Boehmite AlOOH | γ | δ | θ | α-Al2O3 | |||||||||
| Gibbsite Al(OH)3 | χ | κ | α-Al2O3 | ||||||||||
| # Peak | hkl | Center | FWHM | CSR, nm |
|---|---|---|---|---|
| 1 | 111 | 19.56 | 3.3 | 2.4 |
| 2 | 220 | 32.11 | 6.6 | 1.3 |
| 3 | 311 | 37.58 | 4.8 | 1.7 |
| 4 | 222 | 39.73 | 0.9 | 9.0 |
| 5 | 400 | 45.78 | 2.5 | 3.5 |
| 6 | 511 | 61.03 | 5.3 | 1.7 |
| 7 | 440 | 66.81 | 2.6 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsenko, D.A.; Pakharukova, V.P.; Tsybulya, S.V. Low Temperature Transitional Aluminas: Structure Specifics and Related X-ray Diffraction Features. Crystals 2021, 11, 690. https://doi.org/10.3390/cryst11060690
Yatsenko DA, Pakharukova VP, Tsybulya SV. Low Temperature Transitional Aluminas: Structure Specifics and Related X-ray Diffraction Features. Crystals. 2021; 11(6):690. https://doi.org/10.3390/cryst11060690
Chicago/Turabian StyleYatsenko, Dmitriy A., Vera P. Pakharukova, and Sergey V. Tsybulya. 2021. "Low Temperature Transitional Aluminas: Structure Specifics and Related X-ray Diffraction Features" Crystals 11, no. 6: 690. https://doi.org/10.3390/cryst11060690