π-Extended Boron Difluoride [NՈNBF2] Complex, Crystal Structure, Liquid NMR, Spectral, XRD/HSA Interactions: A DFT and TD-DFT Study
Abstract
:1. Introduction
2. Experimental
2.1. Computational
2.2. Materials and Synthesis
2.3. XRD-Structure
3. Results and Discussion
3.1. Preparation and NMR
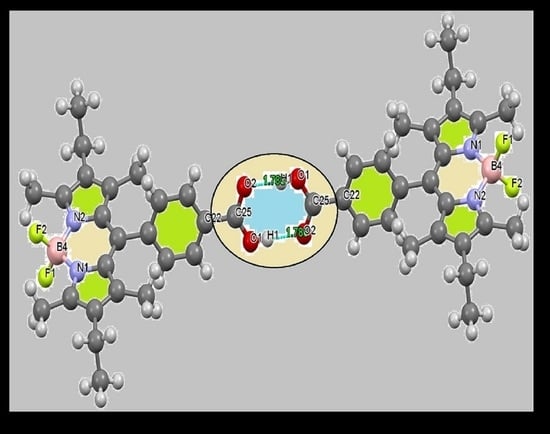
3.2. XRD and DFT
3.3. XRD Packing and HSA Investigation
3.4. MEP, Charges, and GRD Investigations
| I: Ionization potential = −EHOMO A: Electron affinity = −ELUMO ΔΕgap: Energy gap = EHOMO − ELUMO χ: Absolute electronegativity = (I + A)/2 η: Global hardness = (I − A)/2 σ: Global softness = l/η μ: Chemical potential = − χ ω: Electrophilicity = μ2/2η | (1) (2) (3) (4) (5) (6) (7) (8) |
3.5. FT-IR and DFT-IR Spectroscopy
3.6. DOS, HOMO→LUMO, e-transfer/TD-SCF/DFT/B3LYP, and Solvents Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Di-agnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The triplet excited state of Bodipy: Formation, modulation and application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef] [Green Version]
- Nepomnyashchii, A.B.; Bard, A.J. Electrochemistry and Electrogenerated Chemiluminescence of BODIPY Dyes. Accounts Chem. Res. 2012, 45, 1844–1853. [Google Scholar] [CrossRef]
- Bonardi, L.; Ulrich, G.; Ziessel, R. Tailoring the Properties of Boron−Dipyrromethene Dyes with Acetylenic Functions at the 2,6,8 and 4-B Substitution Positions. Org. Lett. 2008, 10, 2183–2186. [Google Scholar] [CrossRef]
- Brizet, B.; Bernhard, C.; Volkova, Y.; Rousselin, Y.; Harvey, P.D.; Goze, C.; Denat, F. Boron functionalization of BODIPY by various alcohols and phenols. Org. Biomol. Chem. 2013, 11, 7729. [Google Scholar] [CrossRef]
- Lakshmi, V.; Rao, M.R.; Ravikanth, M. Halogenated boron-dipyrromethenes: Synthesis, properties and applications. Org. Biomol. Chem. 2015, 13, 2501–2517. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J. Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bio-imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar] [CrossRef]
- Patalag, L.J.; Jones, P.G.; Werz, D.B. BOIMPYs: Rapid Access to a Family of Red-Emissive Fluorophores and NIR Dyes. Angew. Chem. Int. Ed. 2016, 55, 13340–13344. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Rezende, L.C.; Emery, F.S. A review of the synthetic strategies for the development of BODIPY dyes for conjugation with proteins. Orbital Electron. J. Chem. 2013, 5, 62–83. [Google Scholar]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, E.; Galvan-Miranda, E.; Aguilar-Martínez, M.; Farfan, N.; Xochitiotzi-Flores, E.; Ruvalcaba, N. Electroreduction of 8-(thiophen-2-yl)- and 8-(phenyl)-dipyrrometheneboron difluorides. A mechanistic study by cyclic voltammetric digital simulation. Electrochimica Acta. 2019, 317, 375–383. [Google Scholar] [CrossRef]
- Arbeloa, F.L.; Bañuelos, J.; Martínez, V.; Arbeloa, T.; Arbeloa, I.L. Structural, photophysical and lasing properties of pyrromethene dyes. Int. Rev. Phys. Chem. 2005, 24, 339–374. [Google Scholar] [CrossRef]
- Harriman, A. Artificial light-harvesting arrays for solar energy conversion. Chem. Commun. 2015, 51, 11745–11756. [Google Scholar] [CrossRef]
- Findlay, N.J.; Bruckbauer, J.; Inigo, A.R.; Breig, B.; Arumugam, S.; Wallis, D.J.; Martin, R.W.; Skabara, P.J. An Organic Down-Converting Material for White-Light Emission from Hybrid LEDs. Adv. Mater. 2014, 26, 7290–7294. [Google Scholar] [CrossRef] [Green Version]
- Godoy, J.; Vives, G.; Tour, J.M. Synthesis of Highly Fluorescent BODIPY-Based Nanocars. Org. Lett. 2010, 12, 1464–1467. [Google Scholar] [CrossRef]
- Šolomek, T.; Wirz, J.; Klán, P. Searching for Improved Photoreleasing Abilities of Organic Molecules. Accounts Chem. Res. 2015, 48, 3064–3072. [Google Scholar] [CrossRef]
- Rubinstein, N.; Liu, P.; Miller, E.W.; Weinstain, R. meso-Methylhydroxy BODIPY: A scaffold for photo-labile protecting groups. Chem. Commun. 2015, 51, 6369–6372. [Google Scholar] [CrossRef]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1604053. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, W. Syntheses of 2-Aryl Benzothiazoles via Photocatalyzed Oxidative Condensation of Amines with 2-Aminothiophenol in the Presence of BODIPY Derivatives. Synth. Commun. 2014, 44, 3189–3198. [Google Scholar] [CrossRef]
- Majumdar, P.; Yuan, X.; Li, S.; le Guennic, B.; Ma, J.; Zhang, C.; Jacquemin, D.; Zhao, J. Cyclometalated Ir(III) complexes with styryl-BODIPY ligands showing near IR absorption/emission: Preparation, study of photophysical properties and applica-tion as photodynamic/luminescence imaging materials. J. Mater. Chem. B 2014, 2, 2838–2854. [Google Scholar] [CrossRef]
- Mahmood, Z.; Zhao, J. Thiol-Activatable Triplet–Triplet Annihilation Upconversion with Maleimide-Perylene as the Caged Triplet Acceptor/Emitter. J. Org. Chem. 2016, 81, 587–594. [Google Scholar] [CrossRef]
- Sabatini, R.P.; McCormick, T.M.; Lazarides, T.; Wilson, K.C.; Eisenberg, R.; McCamant, D.W. Intersystem Crossing in Halo-genated Bodipy Chromophores Used for Solar Hydrogen Production. J. Phys. Chem. Lett. 2011, 2, 223–227. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, B.; Yang, T.; Yang, Y.; Shen, Z.; Yao, P.; Li, F. A General Strategy for Biocompatible, High-Effective Upconversion Nanocapsules Based on Triplet–Triplet Annihilation. J. Am. Chem. Soc. 2013, 135, 5029–5037. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the bo-ron dipyrromethene core. Chem. Rev. 2019, 399, 213024–213109. [Google Scholar]
- Shipalova, M.V.; Bobrov, A.V.; Usoltsev, S.D.; Marfin, Y.S.; Rumyantsev, E.V. Influence of structure and solvatation on pho-tophysical characteristics of meso-substituted boron dipyrrins in solution and bulk hybrid materials. J. Mol. Liq. 2019, 283, 688–694. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 3.1 Package. University of Western Australia, Perth, Australia 2007. Available online: https://crystalexplorer.scb.uwa.edu.au (accessed on 21 April 2021).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Version 09); Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT– Integrated space-group and crystal-stucture determination. Acta Cryst. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, N.; Freidzon, A.; Vovna, V. Theoretical insights into UV–Vis absorption spectra of difluoroboron β-diketonates with an extended π system: An analysis based on DFT and TD-DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Firmansyah, D.; Yoo, J.; Kumar, R.; Mulugeta, E.; Jo, H.; Ok, K.M.; Lee, C. Fluorescent boron complexes based on new N,O-chelates as promising candidates for flow cytometry. Bull. Korean Chem. Soc. 2017, 38, 1163–1171. [Google Scholar] [CrossRef]
- Banfi, D.; Patiny, L. www.nmrdb.org: Resurrecting and Processing NMR Spectra On-line. Chim. Int. J. Chem. 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Mas-Montoya, M.; Montenegro, M.F.; Ferao, A.E.; Tarraga, A.; Rodríguez-Lopez, J.; Curiel, D. Rigid π-Extended Boron Difluoride Complex with Mega-Stokes Shift for Bioimaging. Org. Lett. 2020, 22, 3356–3360. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Cheng, C.; Yu, C.; Hao, E.; Wang, S.; Wang, J.; Wei, Y.; Mu, X.; Jiao, L. Facile synthesis of highly fluorescent BF2 complexes bearing isoindolin-1-one ligand. Dalton Trans. 2014, 43, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Chang, F.; Wu, Q.; Hao, E.; Jiao, L.; Wang, J.; Pei, J. Synthesis and Semiconducting Characteristics of the BF2 Com-plexes of Bisbenzothiophene-Fused Azadipyrromethenes. Org. Lett. 2020, 22, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, R.; Li, Y.; Li, Q.; Xiao, S. Solid state emission and mechanochromic luminescence of boron 2-(2′-pyridyl)imidazole complexes. Dye. Pigment. 2016, 132, 342–346. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R.; Hammouti, B.; Mouslim, M.; Warad, I. Synthesis of novel Cl2Co4L6 clusterusing 1-hydroxymethyl-3,5-dimethylpyrazole (LH) ligand: Crystal structure, spectral, thermal, Hirschfeld surface analysis and catalytic oxidation evaluation. J. Mol. Struct. 2020, 1199, 126995–127001. [Google Scholar] [CrossRef]
- Titi, A.; Warad, I.; Tillard, M.; Touzani, R.; Messali, M.; El Kodadi, M.; Eddike, D.; Zarrouk, A. Inermolecular interaction in [C6H10N3]2[CoCl4] complex: Synthesis, XRD/HSA relation, spectral and catecholase catalytic analysis. J. Mol. Struct. 2020, 1217, 128422–128431. [Google Scholar] [CrossRef]
- Titi, A.; Oshio, H.; Touzani, R.; Mouslim, M.; Zarrouk, A.; Hammouti, B.; Warad, I. Synthesis and XRD of Novel Ni4(μ3-O)4 Twist Cubane Cluster Using Three NNO Mixed Ligands: Hirshfeld, Spectral, Thermal and Oxidation Properties. J. Clust. Sci. 2021, 32, 227–234. [Google Scholar] [CrossRef]
- Aouad, M.R.; Messali, M.; Rezki, N.; Al-Zaqri, N.; Warad, I. Single proton intramigration in novel 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione: XRD-crystal interactions, physicochem-ical, thermal, Hirshfeld surface, DFT realization of thiol/thione tautomerism. J. Mol. Liq. 2018, 264, 621–630. [Google Scholar] [CrossRef]
- Warad, I.; Suboh, H.; Al-Zaqri, N.; Alsalme, A.; Alharthi, F.A.; Aljohanie, M.M.; Zarrouk, A. Synthesis and physicochemical, DFT, thermal and DNA-binding analysis of a new pentadentate N3S2 Schiff base ligand and it’s [CuN3S2]2+ complexes. RSC Adv. 2020, 10, 21806–21821. [Google Scholar] [CrossRef]
- Chiter, C.; Bouchama, A.; Mouas, T.N.; Allal, H.; Yahiaoui, M.; Warad, I.; Zarrouk, A.; Djedouani, A. Synthesis, crystal struc-ture, spectroscopic and hirshfeld surface analysis, NCI-RDG, DFT computations and antibacterial activity of new asymmet-rical azines. J. Mol. Struct. 2020, 1217, 128376. [Google Scholar] [CrossRef]
- Hema, M.; Warad, I.; Karthik, C.; Zarrouk, A.; Kumara, K.; Pampa, K.; Mallu, P.; Lokanath, N. XRD/DFT/HSA-interactions in Cu(II)Cl/phen/ß-diketonato complex: Physicochemical, solvatochromism, thermal and DNA-binding analysis. J. Mol. Struct. 2020, 1210, 128000–128010. [Google Scholar] [CrossRef]
- Boshaala, A.M.; AlFarha, K.A.; Sheppaek, H.M.; Algezzeri, W.O.; Zarrouk, A.; Warad, I. Crystal interaction, XRD powder, and Hirshfeld surface analysis of S-benzyl-β-N-(1-(4-chlorophenyl) ethylidene) dithiocarbazate Schiff base. Mor. J. Chem. 2020, 8, 1048–1054. [Google Scholar]










| Empirical Formula | C24H27BF2N2O2 |
|---|---|
| Formula weight | 424.28 |
| Temperature/K | 150.0(2) |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 7.6047(3) |
| b/Å | 7.7074(3) |
| c/Å | 21.7634(10) |
| α/° | 89.751(4) |
| β/° | 84.955(3) |
| γ/° | 83.375(3) |
| Volume/Å3 | 1262.16(9) |
| Z, Z‘ | 2, 1 |
| ρcalc g/cm3 | 1.116 |
| μ/mm−1 | 0.660 |
| F(000) | 448.0 |
| Crystal size/mm3 | 0.27 × 0.16 × 0.04 |
| Radiation | CuKα (λ = 1.54184) |
| 2Θ range for data collection/° | 11.558 to 133.652 |
| Index ranges | −9 ≤ h ≤ 8, −9 ≤ k ≤ 9, −25 ≤ l ≤ 25 |
| Reflections collected | 17660 |
| Independent reflections | 4444 [Rint = 0.0465, Rsigma = 0.0367] |
| Data/restraints/parameters | 4444/0/292 |
| Goodness-of-fit on F2 | 1.025 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0452, wR2 = 0.1163 |
| Final R indexes [all data] | R1 = 0.0592, wR2 = 0.1257 |
| Largest diff. peak/hole/e Å−3 | 0.23/−0.23 |
| No. | Bond | XRD | DFT | No. | Bond | XRD | DFT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F1 | B4 | 1.397(2) | 1.4018 | 18 | C5 | C6 | 1.414(3) | 1.4179 | |||||
| 2 | F2 | B4 | 1.389(2) | 1.4017 | 19 | C5 | C15 | 1.493(3) | 1.4927 | |||||
| 3 | O1 | H1 | 0.830(8) | 0.9681 | 20 | C6 | C7 | 1.387(2) | 1.3961 | |||||
| 4 | O1 | C25 | 1.264(3) | 1.3555 | 21 | C6 | C16 | 1.497(3) | 1.5042 | |||||
| 5 | O2 | C25 | 1.263(2) | 1.2074 | 22 | C7 | C10 | 1.434(2) | 1.4341 | |||||
| 6 | N1 | C3 | 1.353(3) | 1.3441 | 23 | C7 | C18 | 1.496(3) | 1.5002 | |||||
| 7 | N1 | C9 | 1.399(2) | 1.3963 | 24 | C8 | C9 | 1.407(2) | 1.4033 | |||||
| 8 | N1 | B4 | 1.543(3) | 1.5531 | 25 | C8 | C10 | 1.389(2) | 1.4033 | |||||
| 9 | N2 | C5 | 1.345(3) | 1.3442 | 26 | C8 | C19 | 1.493(2) | 1.4942 | |||||
| 10 | N2 | C10 | 1.400(2) | 1.3963 | 27 | C12 | C13 | 1.519(4) | 1.5393 | |||||
| 11 | N2 | B4 | 1.543(3) | 1.553 | 28 | C16 | C17 | 1.519(3) | 1.5393 | |||||
| 12 | C1 | C2 | 1.393(3) | 1.3962 | 29 | C19 | C20 | 1.385(2) | 1.3985 | |||||
| 13 | C1 | C9 | 1.419(2) | 1.4341 | 30 | C19 | C24 | 1.397(2) | 1.3994 | |||||
| 14 | C1 | C11 | 1.501(3) | 1.5002 | 31 | C20 | C21 | 1.382(2) | 1.3904 | |||||
| 15 | C2 | C3 | 1.403(3) | 1.4179 | 32 | C21 | C22 | 1.395(2) | 1.3985 | |||||
| 16 | C2 | C12 | 1.505(3) | 1.5042 | 33 | C22 | C23 | 1.392(3) | 1.3982 | |||||
| 17 | C3 | C14 | 1.494(3) | 1.4927 | 34 | C22 | C25 | 1.483(2) | 1.4877 | |||||
| No. | Angles | XRD | DFT | No. | Angles | XRD | DFT | |||||||
| 1 | H1 | O1 | C25 | 117(6) | 106.26 | 28 | C10 | C8 | C19 | 119.0(1) | 119.27 | |||
| 2 | C3 | N1 | C9 | 108.1(1) | 108.89 | 29 | N1 | C9 | C1 | 107.9(1) | 107.55 | |||
| 3 | C3 | N1 | B4 | 126.3(2) | 125.29 | 30 | N1 | C9 | C8 | 119.6(1) | 120.13 | |||
| 4 | C9 | N1 | B4 | 125.6(1) | 125.82 | 31 | C1 | C9 | C8 | 132.5(2) | 132.32 | |||
| 5 | C5 | N2 | C10 | 108.3(1) | 108.89 | 32 | N2 | C10 | C7 | 107.5(1) | 107.55 | |||
| 6 | C5 | N2 | B4 | 126.1(2) | 125.29 | 33 | N2 | C10 | C8 | 120.1(1) | 120.13 | |||
| 7 | C10 | N2 | B4 | 125.4(1) | 125.82 | 34 | C7 | C10 | C8 | 132.4(2) | 132.32 | |||
| 8 | C2 | C1 | C9 | 106.9(1) | 106.73 | 35 | C2 | C12 | C13 | 112.5(2) | 113.71 | |||
| 9 | C2 | C1 | C11 | 124.5(2) | 124.86 | 36 | C6 | C16 | C17 | 112.2(2) | 113.7 | |||
| 10 | C9 | C1 | C11 | 128.7(2) | 128.41 | 37 | C8 | C19 | C20 | 120.7(1) | 120.37 | |||
| 11 | C1 | C2 | C3 | 107.5(2) | 107.26 | 38 | C8 | C19 | C24 | 119.6(1) | 120.42 | |||
| 12 | C1 | C2 | C12 | 127.6(2) | 127.48 | 39 | C20 | C19 | C24 | 119.6(2) | 119.21 | |||
| 13 | C3 | C2 | C12 | 124.9(2) | 125.25 | 40 | C19 | C20 | C21 | 120.5(2) | 120.55 | |||
| 14 | N1 | C3 | C2 | 109.6(2) | 109.57 | 41 | C20 | C21 | C22 | 119.9(2) | 120.01 | |||
| 15 | N1 | C3 | C14 | 122.2(2) | 121.92 | 42 | C21 | C22 | C23 | 119.9(2) | 119.61 | |||
| 16 | C2 | C3 | C14 | 128.1(2) | 128.51 | 43 | C21 | C22 | C25 | 119.9(2) | 122.33 | |||
| 17 | N2 | C5 | C6 | 109.9(2) | 109.57 | 44 | C23 | C22 | C25 | 120.2(2) | 118.06 | |||
| 18 | N2 | C5 | C15 | 122.7(2) | 121.92 | 45 | C22 | C23 | C24 | 119.8(2) | 120.21 | |||
| 19 | C6 | C5 | C15 | 127.4(2) | 128.51 | 46 | C19 | C24 | C23 | 120.2(2) | 120.41 | |||
| 20 | C5 | C6 | C7 | 107.3(1) | 107.26 | 47 | O1 | C25 | O2 | 123.3(2) | 122.26 | |||
| 21 | C5 | C6 | C16 | 125.2(2) | 125.25 | 48 | O1 | C25 | C22 | 118.1(2) | 112.86 | |||
| 22 | C7 | C6 | C16 | 127.4(2) | 127.48 | 49 | O2 | C25 | C22 | 118.6(2) | 124.88 | |||
| 23 | C6 | C7 | C10 | 107.0(1) | 106.73 | 50 | F1 | B4 | F2 | 109.5(2) | 109.76 | |||
| 24 | C6 | C7 | C18 | 124.4(2) | 124.86 | 51 | F1 | B4 | N1 | 109.4(2) | 110.01 | |||
| 25 | C10 | C7 | C18 | 128.6(2) | 128.41 | 52 | F1 | B4 | N2 | 109.5(2) | 110.19 | |||
| 26 | C9 | C8 | C10 | 121.9(1) | 121.48 | 53 | F2 | B4 | N1 | 110.6(2) | 110.19 | |||
| 27 | C9 | C8 | C19 | 119.1(1) | 119.25 | 54 | F2 | B4 | N2 | 110.6(2) | 110.02 | |||
| No. | Dihedral angles | XRD | DFT | No. | Dihedral angles | XRD | DFT | |||||||
| 1 | C9 | N1 | C3 | C2 | 0.5 | −0.22 | 32 | C11 | C1 | C2 | C12 | 1.5 | 0.98 | |
| 2 | C9 | N1 | C3 | C14 | 178.6 | 179.71 | 33 | C2 | C1 | C9 | N1 | 0.4 | 0.07 | |
| 3 | B4 | N1 | C3 | C2 | 179.1 | 179.88 | 34 | C2 | C1 | C9 | C8 | −178 | −179.78 | |
| 4 | B4 | N1 | C3 | C14 | 1.8 | −0.2 | 35 | C11 | C1 | C9 | N1 | 179.4 | 179.99 | |
| 5 | C3 | N1 | C9 | C1 | −0.6 | 0.09 | 36 | C11 | C1 | C9 | C8 | 2.1 | 0.13 | |
| 6 | C3 | N1 | C9 | C8 | 178.1 | 179.97 | 37 | C1 | C2 | C3 | N1 | −0.3 | 0.26 | |
| 7 | B4 | N1 | C9 | C1 | 179.1 | 179.99 | 38 | C1 | C2 | C3 | C14 | 178.8 | 179.65 | |
| 8 | B4 | N1 | C9 | C8 | −2.3 | −0.13 | 39 | C12 | C2 | C3 | N1 | 178 | 179.19 | |
| 9 | C3 | N1 | B4 | F1 | 65.7 | 60.63 | 40 | C12 | C2 | C3 | C14 | −3 | −0.72 | |
| 10 | C3 | N1 | B4 | F2 | −54.9 | −60.52 | 41 | C1 | C2 | C12 | C13 | 86.7 | 89.5 | |
| 11 | C3 | N1 | B4 | N2 | −175.5 | −179.89 | 42 | C3 | C2 | C12 | C13 | −91.2 | −89.21 | |
| 12 | C9 | N1 | B4 | F1 | −113.8 | −119.26 | 43 | N2 | C5 | C6 | C7 | 1.3 | 0.22 | |
| 13 | C9 | N1 | B4 | F2 | 125.5 | 119.59 | 44 | N2 | C5 | C6 | C16 | 178.6 | 179.12 | |
| 14 | C9 | N1 | B4 | N2 | 4.9 | 0.22 | 45 | C15 | C5 | C6 | C7 | −177 | −179.69 | |
| 15 | C10 | N2 | C5 | C6 | −1.4 | −0.18 | 46 | C5 | C6 | C7 | C18 | 177 | 179.85 | |
| 16 | C10 | N2 | C5 | C15 | 177 | 179.73 | 47 | C16 | C6 | C7 | C10 | 177 | 179.03 | |
| 17 | B4 | N2 | C5 | C6 | 174.3 | 179.81 | 48 | C6 | C7 | C10 | C8 | −177 | −179.9 | |
| 18 | B4 | N2 | C5 | C15 | −7.3 | −0.27 | 49 | C18 | C7 | C10 | N2 | −177 | −179.96 | |
| 19 | C5 | N2 | C10 | C7 | 1 | 0.08 | 50 | C10 | C8 | C9 | C1 | 177 | 179.8 | |
| 20 | C5 | N2 | C10 | C8 | 179.4 | 179.96 | 51 | C19 | C8 | C9 | N1 | 177 | 179.95 | |
| 21 | B4 | N2 | C10 | C7 | −174.8 | −179.92 | 52 | C9 | C8 | C10 | C7 | −177 | −179.96 | |
| 22 | B4 | N2 | C10 | C8 | 3.6 | 0.04 | 53 | C19 | C8 | C10 | N2 | −177 | −179.9 | |
| 23 | C5 | N2 | B4 | F1 | −61.9 | −60.81 | 54 | C9 | C8 | C19 | C20 | −177 | −88.83 | |
| 24 | C5 | N2 | B4 | F2 | 58.8 | 60.35 | 55 | C10 | C8 | C19 | C20 | 177 | 91.17 | |
| 25 | C5 | N2 | B4 | N1 | 179.4 | 179.82 | 56 | C10 | C8 | C19 | C24 | −177 | −88.84 | |
| 26 | C10 | N2 | B4 | F1 | 113.1 | 119.19 | 57 | C8 | C19 | C20 | C21 | −177 | −179.97 | |
| 27 | C10 | N2 | B4 | F2 | −126.2 | −119.66 | 58 | C8 | C19 | C24 | C23 | −177 | −179.93 | |
| 28 | C10 | N2 | B4 | N1 | −5.5 | −0.18 | 59 | C20 | C21 | C22 | C25 | −177 | −179.94 | |
| 29 | C9 | C1 | C2 | C3 | −0.1 | −0.2 | 60 | C25 | C22 | C23 | C24 | −177 | −179.96 | |
| 30 | C9 | C1 | C2 | C12 | −178.3 | −179.1 | 61 | C21 | C22 | C25 | O2 | 177 | 179.86 | |
| 31 | C11 | C1 | C2 | C3 | 179.8 | 179.88 | 62 | C23 | C22 | C25 | O1 | 177 | 179.86 | |
| No. | Atom | MAC | NPA | No. | Atom | MAC | NPA |
|---|---|---|---|---|---|---|---|
| 1 | F | −0.29709 | −0.53585 | 30 | H | 0.091309 | 0.18918 |
| 2 | F | −0.29348 | −0.53248 | 31 | H | 0.13651 | 0.2186 |
| 3 | O | −0.26969 | −0.62354 | 32 | C | −0.18864 | −0.58366 |
| 4 | H | 0.265325 | 0.46816 | 33 | H | 0.131435 | 0.2136 |
| 5 | O | −0.37872 | −0.63625 | 34 | H | 0.094352 | 0.19069 |
| 6 | N | −0.43505 | −0.56227 | 35 | H | 0.133131 | 0.22047 |
| 7 | N | −0.4279 | −0.55671 | 36 | C | −0.2127 | −0.3594 |
| 8 | C | −0.10506 | 0.02207 | 37 | H | 0.113849 | 0.18236 |
| 9 | C | −0.19772 | −0.09985 | 38 | H | 0.116174 | 0.18306 |
| 10 | C | 0.17403 | 0.32414 | 39 | C | −0.2293 | −0.51552 |
| 11 | C | 0.176512 | 0.33589 | 40 | H | 0.098476 | 0.1797 |
| 12 | C | −0.19924 | −0.09794 | 41 | H | 0.099777 | 0.17848 |
| 13 | C | −0.09659 | 0.03016 | 42 | H | 0.097746 | 0.17814 |
| 14 | C | −0.07529 | 0.03442 | 43 | C | −0.16669 | −0.56563 |
| 15 | C | 0.257276 | 0.11308 | 44 | H | 0.126768 | 0.20287 |
| 16 | C | 0.253713 | 0.11242 | 45 | H | 0.100512 | 0.19281 |
| 17 | C | −0.16645 | −0.56321 | 46 | H | 0.120702 | 0.20111 |
| 18 | H | 0.127112 | 0.20256 | 47 | C | −0.23101 | −0.00949 |
| 19 | H | 0.098994 | 0.19184 | 48 | C | −0.03429 | −0.16719 |
| 20 | H | 0.116622 | 0.19892 | 49 | H | 0.094639 | 0.18222 |
| 21 | C | −0.21392 | −0.35705 | 50 | C | 0.009118 | −0.12842 |
| 22 | H | 0.11481 | 0.18151 | 51 | H | 0.095902 | 0.18206 |
| 23 | H | 0.114148 | 0.18229 | 52 | C | −0.22343 | −0.14409 |
| 24 | C | −0.22818 | −0.51663 | 53 | C | 0.010034 | −0.10858 |
| 25 | H | 0.100367 | 0.17925 | 54 | H | 0.106701 | 0.19256 |
| 26 | H | 0.095844 | 0.1774 | 55 | C | −0.03609 | −0.16044 |
| 27 | H | 0.097458 | 0.17899 | 56 | H | 0.097616 | 0.18146 |
| 28 | C | −0.19803 | −0.58381 | 57 | C | 0.400776 | 0.79126 |
| 29 | H | 0.133892 | 0.21604 | 58 | B | 0.502918 | 1.19828 |
| GRD | Value | |
|---|---|---|
| Global total energy | ET | −1415.9090 a.u, |
| Low unoccupied molecular orbital | LUMO | −0.0838 a.u. |
| High occupied molecular orbital | HOMO | −0.1917 a.u. |
| Energy gap | ΔEgap | 0.1080 a.u. 2.941 eV |
| Electron affinity | A | 2.2803 eV |
| Ionization potential | I | 5.2164 eV |
| Global hardness | ƞ | 2.9361 eV |
| Global softness | σ | 0.3406 eV |
| Chemical potential | μ | −3.7484 eV |
| Absolute electronegativity | X | 3.7484 eV |
| Electrophilicity | ω | 2.3927 eV |
| Dipole Moment | ᶙ | 3.0497 D |
| No. | λmax (nm) | Osc. Str. (f) | Major Contributions |
|---|---|---|---|
| 1 | 433.49 | 0.3433 | HOMO->LUMO (84%) |
| 2 | 389.14 | 0.0127 | H-1->LUMO (34%), HOMO->L+1 (65%) |
| 3 | 367.63 | 0.2794 | H-1->LUMO (56%), HOMO->LUMO (16%), HOMO->L+1 (26%) |
| 4 | 354.34 | 0.0495 | H-2->LUMO (97%) |
| 5 | 308.29 | 0.0034 | HOMO->L+2 (97%) |
| 6 | 296.94 | 0.009 | H-1->L+1 (98%) |
| 7 | 295.84 | 0.113 | H-3->LUMO (88%) |
| 8 | 287.27 | 0.0002 | H-4->LUMO (45%), H-4->L+1 (51%) |
| 9 | 282.25 | 0.0012 | H-2->L+1 (99%) |
| 10 | 277.55 | 0.0027 | H-5->LUMO (97%) |
| 11 | 259.69 | 0.0016 | H-4->LUMO (54%), H-4->L+1 (42%) |
| 12 | 255.00 | 0.2109 | H-6->LUMO (73%), H-3->L+1 (14%) |
| No. | λmax (nm) | Osc. Str. (f) | Major Contributions |
|---|---|---|---|
| 1 | 409.5942947 | 0.5877 | HOMO->LUMO (95%) |
| 2 | 326.47 | 0.1111 | H-1->LUMO (93%) |
| 3 | 306.38 | 0.0594 | H-2->LUMO (95%) |
| 4 | 290.05 | 0.0263 | HOMO->L+1 (95%) |
| 5 | 258.94 | 0.0032 | H-6->L+1 (76%), H-6->L+4 (10%) |
| 6 | 254.11 | 0.2336 | H-3->LUMO (93%) |
| 7 | 240.04 | 0.0105 | H-4->LUMO (51%), H-4->L+1 (29%), H-3->L+2 (15%) |
| 8 | 237.18 | 0.0224 | HOMO->L+2 (98%) |
| 9 | 224.29 | 0.3417 | H-5->LUMO (77%), H-3->L+1 (12%) |
| 10 | 221.17 | 0.0029 | H-4->LUMO (39%), H-4->L+1 (28%), H-1->L+1 (23%) |
| 11 | 218.89 | 0.0033 | H-4->L+1 (12%), H-1->L+1 (72%) |
| 12 | 213.61 | 0.116 | H-5->LUMO (13%), H-3->L+1 (64%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsimaree, A.A.; Alsenani, N.I.; Alatawi, O.M.; AlObaid, A.A.; Knight, J.G.; Messali, M.; Zarrouk, A.; Warad, I. π-Extended Boron Difluoride [NՈNBF2] Complex, Crystal Structure, Liquid NMR, Spectral, XRD/HSA Interactions: A DFT and TD-DFT Study. Crystals 2021, 11, 606. https://doi.org/10.3390/cryst11060606
Alsimaree AA, Alsenani NI, Alatawi OM, AlObaid AA, Knight JG, Messali M, Zarrouk A, Warad I. π-Extended Boron Difluoride [NՈNBF2] Complex, Crystal Structure, Liquid NMR, Spectral, XRD/HSA Interactions: A DFT and TD-DFT Study. Crystals. 2021; 11(6):606. https://doi.org/10.3390/cryst11060606
Chicago/Turabian StyleAlsimaree, Abdulrahman A., Nawaf. I. Alsenani, Omar Mutlaq Alatawi, Abeer A. AlObaid, Julian Gary Knight, Mouslim Messali, Abdelkader Zarrouk, and Ismail Warad. 2021. "π-Extended Boron Difluoride [NՈNBF2] Complex, Crystal Structure, Liquid NMR, Spectral, XRD/HSA Interactions: A DFT and TD-DFT Study" Crystals 11, no. 6: 606. https://doi.org/10.3390/cryst11060606