1. Introduction
Cobalt-based alloys have existed since the first half of the last century. The two main applications were prosthetic dentistry [
1] and high temperature components in aeroengines [
2]. Early on, nickel was present in many compositions of cobalt alloys, even in dental alloys (e.g., Vitallium, Co–27Cr–5.5Mo–2.5Ni–0.25C). In the last decades, nickel was removed from many “predominantly base” alloys because of the possibility of induced allergic diseases. In contrast, this element still features in most of cobalt-based superalloys [
3]. Its role is notably favoring the austenitic structure of cobalt alloys at medium temperatures, by hindering the allotropic transformation to hexagonal compact [
4]. There are also several cobalt-based alloys which do not contain nickel, Mar-M 302 or WI-52 for instance. Because of its melting point—which is about 40 °C lower than the cobalt’s one (1455 against 1495 °C)—nickel may be suspected to lower a little the refractoriness of some cobalt-based alloys. Nickel is an important element in the chemical compositions of some of the famous low-expansion alloys named Invar [
5]. The presence of a lot of chromium in cobalt-based superalloys for increasing the high temperature oxidation and corrosion resistances [
6] may induce an enhanced thermal expansion for them [
7,
8]. For constant Cr content in the alloy, additions of nickel may possibly correct partially this chromium effect. Another important property for the use of metallic materials at high temperature in gaseous environments that are generally chemical aggressive is the resistance to hot oxidation and hot corrosion. It has been known for a long time that the behavior of nickel-free alloys with cobalt and chromium as the main elements are not very good. Several Cr contents were very early tested in binary Co–Cr alloys, for example by Kofstad [
9,
10,
11,
12] in the 10–35 wt % Cr range, for exploring the behavior variation of these alloys in oxidation at 800 to 1300 °C versus the Cr content. This demonstrated that the Cr content must be present at the highest contents tested in these works to allow a real protection of such alloys by the development of a continuous chromia scale. Unfortunately, as consequences, such high Cr amounts have not only the deleterious effect noted above concerning thermal expansion but also possible decreases in refractoriness and metallurgical instabilities (precipitation of brittle TCP-type phases). Nickel additions to cobalt-based alloys are recognized to facilitate volume chromium diffusion which can be of great help in parallel with grain boundary chromium diffusion which can be insufficient in severe conditions. In this way, Ni may allow chromium to be present in not too high contents in the alloy.
The present work is focused on a particular cobalt-based alloy. Its composition is Co(bal.)–25Cr–0.4C–6Ta, all contents being in weight percent. Due to its particularly high tantalum and carbon contents (weight contents rated in order to have the same atomic contents in Ta and in C), this alloy benefits from both a matrix mechanically resistant at high temperature and an interesting interdendritic carbides network. This one is exclusively made of script-like eutectic TaC carbides, efficient for maintaining the interdendritic cohesion for a long time at elevated temperature. Unfortunately, the oxidation resistance of this alloy in air at temperatures high enough to possibly reveal its superior mechanical strength is not good enough to really test it in conditions close to an industrial application. Increasing its chromium content beyond the 25 wt % is potentially dangerous because of the risk of TCP precipitation due to the rather high Ta content.
A possible way to improve its oxidation behavior without resorting to coatings (which can be difficult to efficiently deposit on the surface of complex-shaped components), is to add nickel to take benefit from an easier Cr diffusion. Another possible—but not really expected—effect may be a slightly lowered thermal expansion coefficient. At the same time, it is unfortunately possible to observe detrimental effects such as a decrease in refractoriness.
In this work a new ingot of the Co(bal.)–25Cr–0.4C–6Ta composition will be elaborated, as well as two ingots of nickel-added versions (with 13.7 wt % Ni and 27.4 wt % Ni), following the same fabrication procedure. The three alloys will be subjected to differential scanning calorimetry (DSC), to thermo-mechanical analysis (TMA) and to thermogravimetry (TG) for oxidation. This will allow verification of whether such Ni additions really improve the oxidation behavior as well as the control of their consequences regarding the two other properties under interest.
2. Materials and Methods
2.1. From Elaboration to Samples
The three alloys were prepared by high frequency induction melting under 300 mbars of pure Ar (HF furnace, CELES, France), from small parts of pure elements (Co, Ni, Cr, Ta and graphite rods from Alfa Aesar). The obtained ovoid-shaped ingots, with a mass of about 40 g, were first cut in different parts using an abrasive cutter. They were then machined with a metallographic saw to prepare the samples for the different examinations and tests. The dimensions of each sample were optimized to obtain the best compromise between the dimensional sample possibilities for each thermal test apparatus and the necessity to have enough material to be representative of the behavior of the whole alloy (DSC and TMA) and of its surface (TG). With volumes all higher than 20 mm3 and external surfaces of more than 2 cm2 the results will be independent of the microstructure texture (mix of various structural orientation and microstructure fineness of about 20 µm). This allowed only one test to be performed by thermal characterization.
2.2. Control of the Chemical Compositions, As–Cast Microstructures and Hardnesses
The chemical compositions were measured on embedded, ground and polished mirror-like metallographic preparations, using the energy dispersion spectrometer (EDS) attached to a scanning electrons microscope (JSM-6010LA, JEOL, Tokyo, Japan). With this SEM, the microstructures were observed in back scattered electrons mode (BSE). The acceleration voltage for all these analyses and observations was 20 kV.
The characterization of the as-cast states of the three alloys was completed by performing Vickers indentation three times per alloy. This was done using a macro–indentor (Testwell Wolpert, Germany) machine with a load equal to 10 kg.
2.3. Assessment of the Melting and Solidification Temperature Ranges
A 2 × 2 × 5 mm3 parallelepiped alloy part was placed in a small alumina crucible. These dimensions are almost the maximal ones to be compatible with the alumina crucible capacity. A thermobalance coupled with a differential thermal analyzer (TG-ATD 92.16-18, SETARAM, Caluire, France) was used for performing DSC runs to determine the temperatures of melting start, melting end, solidification start and solidification end. The first applied cycle—heating at +20 °C min−1 up to 1200 °C and at +5 °C min−1 up to 1500 °C followed by cooling down to 1200 °C at −5 °C min−1—aimed to specify these temperatures values for the as-cast microstructure resulting from the rapid solidification in contact with the cold metallic crucible of the HF furnace. It was followed by a second cycle—heating from 1200 to 1500 °C at +5 °C min−1 and cooling from 1500 to 1200 °C at −5 °C min−1—to measure the same temperatures but for a version of the alloy which solidified much slower than during the elaboration with the HF furnace. Finally, cooling was done between 1200 °C and ambient temperature at −20 °C min−1.
2.4. Behaviors in Thermal Expansion and Thermal Contraction; Dimensional Stability at Elevated Temperature
A 7 × 7 × 3 (expansion direction) mm3 parallelepiped alloy part was prepared to characterize the dimensional behavior of the alloy during heating up to a high temperature, during an isothermal stay at this high temperature, and during the cooling down to ambient temperature. The dimensions of the samples are close to those that are recommended for the used thermo–dilatometer (TMA 92.16-18, SETARAM, Caluire, France). Heating up to 1250 °C and cooling down to ambient temperature were carried out at +10 °C min−1 and −10 °C min−1 respectively. The two were separated from one another by a stage at 1250 °C applied for 1 h. The thermo–mechanical analysis (TMA) runs allowed observing possible phase transformations, and at least assessing the values of the thermal expansion coefficients (TEC) at heating and at cooling. The 1 h of isothermal exposure between heating and cooling allowed observation of the possible evolution of the thickness of the samples due to phase transformation or stress release.
2.5. Kinetic of Oxidation at High Temperature and Oxide Scale Behavior during Cooling
An 8 × 8 × 3 mm3 parallelepiped alloy part (maximal dimensions compatible with the hot zone size) was ground with 1200-grit SiC papers to initialize all the six faces. The same papers were used to smooth edges and corners for avoiding any too early local catastrophic oxidation. Smoothing edges and corners also allows limiting stresses in the external oxide during the cooling to do not facilitate scale spallation at cooling. The sample was hung, with an alumina-gained platinum wire, in the hot zone of a thermo–balance (TGA 92.16-18, SETARAM, France). The heating, isothermal stage and cooling were carried out in a 1.5 L h−1 flow of dry synthetic air (80% N2–20% O2). Heating was performed at +20 °C min−1 up to 1250 °C, the temperature at which the sample stayed thereafter during 70 h. At the end of this isothermal stage, a cooling at −5 °C min−1 down to ambient temperature was applied. The low level of this cooling rate aimed to prevent the most possible oxide scale spallation. In the recorded mass variation files obtained with the three TG runs:
The heating part was kept for studying the oxidation progress during the heating (correction from air buoyancy variation and procedure described in [
13]);
The isothermal part was classically exploited to kinetically characterize isothermal oxidation (after correction from chromia volatilization by adding a (Kv × t) term to the mass gain, with Kv taken equal to 200 × 10
−10 g cm
−2 s
−1 [
14]);
The cooling part was kept for studying the oxide scale spallation progress (correction from air buoyancy variation and procedure described in [
15]).
2.6. Metallographic Examination of the Oxidized Samples
After the TG tests, the oxidized samples were carefully removed from the ceramic–gained platinum wire. After deposition of gold all around the sample (to give electrical conductivity to its surface), achieved using a cathodic sprayer (JFC–1200, JEOL, Tokyo, Japan), the sample was coated by electrolytic nickel deposition to obtain a protective metallic shell devoted to the protection of the external scales during cutting. The sample was cut to obtain two equivalent halves which were embedded (resin CY230 and hardener HY956, ESCIL, France), ground and polished to obtain a mirror–like state. The obtained cross–sections were examined and analyzed with the SEM and its EDS spectrometer.
4. Discussion
The possible effects of the addition of nickel were expected first for the microstructures of the alloys resulting from solidification. Obviously, even for the highest Ni quantity added in this work, there is no change in the microstructure of the alloys. The CBLT and its Ni-added versions are totally similar. The rare chromium carbides which appeared at solidification for 27.4 wt % Ni added, obviously disappeared at service temperature and did not re-precipitate at cooling despite the low cooling rate. However, the transient appearance of chromium carbides let think that more abundant and stable chromium carbides may appear for nickel contents high enough. This seems occurring for content a little beyond 28 wt %, and may be as soon as Ni becomes more present than Co (i.e., near 35 wt %Ni). An earlier study showed that Cr
7C
3 are present in higher fractions than TaC in Ni–30Cr–0.4C–6Ta [
16].
If the as-cast microstructures seem to be almost invariant versus the Ni content, this is, in contrast, not the case of the room temperature hardness. This one clearly decreases with Ni addition, an evolution which can be easily explained by the well-known difference in intrinsic hardness of solid pure cobalt (hard) and solid pure nickel (softer than Co).
The DSC runs showed that the additions of nickel influence the melting range of the alloys, and not in the expected direction. Indeed, the average value of the two temperatures associated to the solidus one (i.e., the temperatures of melting start and of solidification end) was increased by nickel addition. At the same time the average temperature associated to the liquidus one also varied a little but not monotonously. Globally the melting temperature range became less extended and the two peaks (both endothermic or both exothermic) started to be closely mixed, this resulting in an apparent single peak. One logical guess is that the first endothermic peak corresponds to the melting of the (matrix and TaC)-eutectic compound (and the second exothermic peak is the solidification of this compound). The second endothermic peak corresponds to the melting of the dendrites of solid solution (and the first exothermic peak results from their crystallization). With 27.4 wt % Ni added, dendrites start melting when the eutectic compound is far from having finished its fusion. However, the double nature of the single peak is still visible, thanks to a bump on one of the peak sides. Since there is almost only a single peak for both melting and solidification for the third alloy, the temperature melting range is considerably less extended than for the two first alloys. This is beneficial for foundry practice and for the foundry health. Indeed, a less extended mushy zone disadvantages the appearance of shrinkage defects in the last zones to solidify.
Concerning the double-cycled DSC tests which were carried out in this work the interest of running a second test after the first one was demonstrated. The first cycle was very useful for re-initializing the alloy with the slow solidification occurring during the slow cooling part of the first cycle. The second heating curve was much less perturbed than the first one and thus able to give more accurate values of the critical temperatures of interest.
One also noticed that the start of solidification was, for all alloys, a little earlier for the second cycle than for the first cycle. This suggests that more nuclei were present in the melt after two cycles rather than one cycle. This tends to reduce undercooling and then this leads to solidification start temperatures which are closer to the liquidus temperatures of the alloys.
To finish with the DSC test, one must try to find possible explanation for the very curious effect of the Ni addition on the average temperature associated to the solidus, and interpret this results in term of practical consequences. By simply considering the melting points order between cobalt and nickel this was more a decrease which may be expected. In the case of Co–Ni binary alloys, it is clear that such results would be wrong. In the present case, even if the alloys are not very complex chemically (quaternary and quinary systems), the presence of chromium, carbon and tantalum in rather high quantities may influence this refractoriness hierarchy. However, a calculated isopleth section of the Co–Ni –Cr–C–Ta diagram, presented in
Figure 11, does not show such effect of nickel for the alloy base under study here. Indeed, according to these thermodynamic calculations, adding Ni ought to decrease the solidus temperature. However, it is true that this system is rather complex and that the used database is not complete (some subsystems are still lacking). Anyway, if this increase in melting start temperature with Ni addition is real, this is an unexpected potentially good point for the high temperature capability of the alloy.
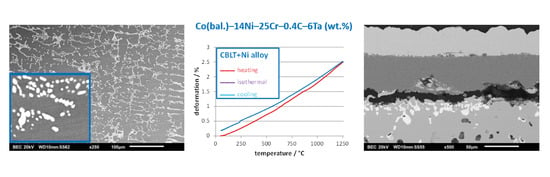
The second thermal experiment which was done here for each alloy was TMA. The first observation concerned the average thermal expansion coefficient. Seemingly it tends decreasing a little when nickel is added. This very moderate effect (average of 0.4 × 10
−6 °C
−1 less by 14 wt % Ni–added slice) may contribute to better behavior in thermal cycling concerning the induced thermal stresses. This may be a second good point for the addition of nickel. In addition, with 27 wt % Ni added, the deformation is almost totally reversible while a persistent positive deformation stayed after thermal cycling for the two CBLT and CBLT+Ni alloys. When one looked to the dimensional behavior of the samples during the 1 h-stage at 1250 °C separating the heating phase and the cooling phase, one noticed that the thicknesses of the CBLT and CBLT+Ni samples tended to go on increasing a little. One can remind that such phenomenon was earlier encountered for 30 wt % Cr-containing (Ni, Co or Fe-based) alloys moderately [
8] or very [
17] rich in carbon then in chromium carbides. This was also observed for 30 wt % Cr-containing Co-based alloys [
18] or Fe-based alloys [
19] rich in tantalum carbides. In these previous studies the isothermal deformations, which were much more marked than here, were interpreted by increasing internal mechanical interactions acting between matrix and carbides. For instance, during the heating phase, the metallic matrixes tended to expanse much more than carbides because of the difference in thermal expansion coefficient (for matrix: two times higher than for carbides). This led to carbides more and more elastically deformed by the increasing tensile stresses applied by matrix and to matrix more and more elastically deformed by the increasing compressive stress applied by carbides. When the temperature had become high enough, the matrix began to be plastically, then visco-plastically, deformed under the compressive action of the carbides. The results of that were the slowdown of the expansion of the whole alloy, then possibly a stationary state and even an inversion (contraction) although that temperature went on increasing. At the end of heating, during the isothermal stage, the compressive deformation suddenly accelerated then slowed down and finished to stabilize. In the present case, the alloys are far from these extreme behaviors because of carbides networks not as dense and interconnected as in the alloys of the previously reported results. It seems that this is another phenomenon. At the end of heating, the thermal stress state of the alloys is much lower, but the internal stresses applied by the matrix and carbides to each other certainly exist too. At this very elevated temperature the carbides may start fractioning, and give a little freedom to matrix, with as result a very slow isothermal additional elongation.
Concerning oxidation, the first point of interest was the moment, during the heating phase, at which oxidation starts to be significant in term of mass gain (according to the sensitivity or accuracy of the used thermo–balance). The results were a little scattered in term of dependence on the Ni content. Indeed, no clear dependence appeared, neither for oxidation start temperature, nor of total weight gain during the whole heating. It is possible that other parameters can be of higher importance for these very early times of oxidation. One can think to the microstructural characteristics of the alloy surface and subsurface (local dendritic orientation, surface state and reactivity a little different between samples, etc.) and the consequences on the oxide nucleation. It was also seen that the later the oxidation start (i.e., the higher the temperature of oxidation start) the faster the subsequent mass gain (e.g., CBLT+Ni by comparison to the two other alloys). If oxidation starts at rather low temperature, one can imagine that oxide nucleation can be done in many locations with consequently the possibility to have thereafter a thin oxide film almost continuous and then a low growth rate for the first scales. On the contrary, if oxidation starts much later, less numerous nuclei can be obtained, and the formed scales can be much discontinuous. Some consequences of that are higher surface fraction for linear growth kinetic, and lower surface fraction for parabolic growth kinetic, than for an alloy with oxidation start much earlier.
This is at the isothermal oxidation kinetic level that nickel addition demonstrated positive consequences. First, the two nickel-richest alloys oxidized according to a mass gain parabolic law over the whole test duration while the nickel-free alloy started linear oxidation in the middle of the 70 h. Second, the (chromia volatilization)-corrected parabolic constant, rather high due to the exceptional highness of the test temperature, decreases when the nickel content in alloy increases. However, this Kp remains significantly higher than the obtained value for the highly oxidation-resistant Ni–30 wt % Cr alloy the (chromia volatilization)-corrected whose Kp is much lower, even at a higher temperature (245 × 10−12 g−2 cm−4 s−1 at 1300 °C).
Concerning the sustainability of the oxide scale during cooling, problems occurred rather early (near 1000 °C for all alloys), despite the low cooling rate. It is sure that, in case of much faster cooling as this can be encountered in practical situation, spallation should surely occur at higher temperature. It is often suspected that the growth of subsurface islands of CrTaO4 oxides—promoted by the high content in tantalum of these alloys—can be responsible of a bad adherence of the chromia scale formed on the alloys. Thus, the loss of the protective scales as soon as the component decreases in temperature, forces the alloys to reconstitute their protective scales. The higher chromium diffusion easiness given by the presence of nickel may be of great importance for facilitating the supply in chromium of the oxidation front. This is another good point for the nickel addition.