Numerical Simulation of Interaction between Kr+ Ion and Rotating C60 Fullerene towards for Nanoarchitectonics of Fullerene Materials
Abstract
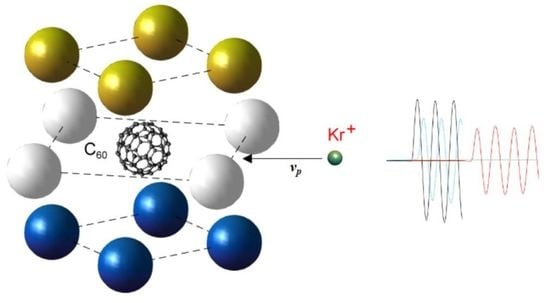
:1. Introduction
2. Physical Statement of the Problem
3. Mathematical Statement of the Problem
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: What’s Coming next after Nanotechnology? Nanoscale Horiz. 2021, 6, 364–378. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for Hierarchical Fullerene Nanomaterials. Nanomaterials 2021, 11, 2146. [Google Scholar] [CrossRef]
- Neal, E.A.; Nakanishi, T. Alkyl-Fullerene Materials of Tunable Morphology and Function. Bull. Chem. Soc. Jpn. 2021, 94, 1769–1788. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, R.G.; Lee, J.; Han, S.A.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Macaroni Fullerene Crystals-Derived Mesoporous Carbon Tubes as a High Rate Performance Supercapacitor Electrode Material. Bull. Chem. Soc. Jpn. 2021, 94, 1502–1509. [Google Scholar] [CrossRef]
- Terrones, H.; Terrones, M. Curved Nanostructured Materials. New J. Phys. 2003, 5, 126. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical Engineers Get to Revisit an Old Friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef] [Green Version]
- Veclani, D.; Tolazzi, M.; Melchior, A. Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments. Processes 2020, 8, 642. [Google Scholar] [CrossRef]
- Wilson, M.A.; Pang, L.S.K.; Willett, G.D.; Fisher, K.J.; Dance, I.G. Fullerenes—Preparation, Properties, and Carbon Chemistry. Carbon 1992, 30, 675–693. [Google Scholar] [CrossRef]
- Neyts, E.; Maeyens, A.; Pourtois, G.; Bogaerts, A. A Density-Functional Theory Simulation of the Formation of Ni-Doped Fullerenes by Ion Implantation. Carbon 2011, 49, 1013–1017. [Google Scholar] [CrossRef]
- Makarets, M.V.; Prylutskyy, Y.I.; Ogloblya, O.V.; Carta-Abelmann, L.; Scharff, P. Computer Simulation of Supported C60 Fullerenes Fragmentation by Particle Beam. Carbon 2004, 42, 987–990. [Google Scholar] [CrossRef]
- Tellgmann, R.; Krawez, N.; Lin, S.-H.; Hertel, I.V.; Campbell, E.E.B. Endohedral Fullerene Production. Nature 1996, 382, 407–408. [Google Scholar] [CrossRef]
- Pietzak, B.; Waiblinger, M.; Murphy, T.A.; Weidinger, A.; Höhne, M.; Dietel, E.; Hirsch, A. Buckminsterfullerene C60: A Chemical Faraday Cage for Atomic Nitrogen. Chem. Phys. Lett. 1997, 279, 259–263. [Google Scholar] [CrossRef]
- Heath, J.R.; O’Brien, S.C.; Zhang, Q.; Liu, Y.; Curl, R.F.; Tittel, F.K.; Smalley, R.E. Lanthanum Complexes of Spheroidal Carbon Shells. J. Am. Chem. Soc. 1985, 107, 7779–7780. [Google Scholar] [CrossRef]
- Guo, T.; Diener, M.D.; Chai, Y.; Alford, M.J.; Haufler, R.E.; McClure, S.M.; Ohno, T.; Weaver, J.H.; Scuseria, G.E.; Smalley, R.E. Uranium Stabilization of C28: A Tetravalent Fullerene. Science 1992, 257, 1661–1664. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Lebedkin, S.; Renker, B.; Heid, R.; Schober, H.; Rietschel, H. A Spectroscopic Study of M@C82 Metallofullerenes: Raman, Far-Infrared, and Neutron Scattering Results. Appl. Phys. Mater. Sci. Process. 1998, 66, 273–280. [Google Scholar] [CrossRef]
- Saunders, M.; Jimenez-Vazquez, H.A.; Cross, R.J.; Mroczkowski, S.; Gross, M.L.; Giblin, D.E.; Poreda, R.J. Incorporation of Helium, Neon, Argon, Krypton, and Xenon into Fullerenes Using High Pressure. J. Am. Chem. Soc. 1994, 116, 2193–2194. [Google Scholar] [CrossRef]
- Saunders, M.; Cross, R.J.; Jimenez-Vazquez, H.A.; Shimshi, R.; Khong, A. Noble Gas Atoms Inside Fullerenes. Science 1996, 271, 1693–1697. [Google Scholar] [CrossRef]
- DiCamillo, B.A.; Hettich, R.L.; Guiochon, G.; Compton, R.N.; Saunders, M.; Jiménez-Vázquez, H.A.; Khong, A.; Cross, R.J. Enrichment and Characterization of a Noble Gas Fullerene: Ar@C60. J. Phys. Chem. 1996, 100, 9197–9201. [Google Scholar] [CrossRef]
- Brink, C.; Hvelplund, P.; Shen, H.; Jiménez-Vázquez, H.A.; Cross, R.J.; Saunders, M. Collisional Fragmentation of Ar@C60. Chem. Phys. Lett. 1998, 286, 28–34. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Ohno, K.; Shiga, K.; Kawazoe, Y.; Maruyama, Y.; Masumoto, K. Insertion of Xe and Kr Atoms into C60 and C70 Fullerenes and the Formation of Dimers. Phys. Rev. Lett. 1998, 81, 967–970. [Google Scholar] [CrossRef]
- Gadd, G.E.; Evans, P.J.; Hurwood, D.J.; Morgan, P.L.; Moricca, S.; Webb, N.; Holmes, J.; McOrist, G.; Wall, T.; Blackford, M.; et al. Endohedral Fullerene Formation through Prompt Gamma Recoil. Chem. Phys. Lett. 1997, 270, 108–114. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, A.; Singh, F.; Kabiraj, D.; Avasthi, D.K.; Pivin, J.C. Ion Irradiation Induced Surface Modification Studies of Polymers Using SPM. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 186–194. [Google Scholar] [CrossRef]
- Narayanan, K.L.; Kojima, N.; Yamaguchi, K.; Ishikawa, N.; Yamaguchi, M. Arsenic Ion Implantation Induced Structural Effects in C60 Films. J. Mater. Sci. 1999, 34, 5227–5231. [Google Scholar] [CrossRef]
- Todorović-Marković, B.; Draganić, I.; Vasiljević-Radović, D.; Romčević, N.; Blanuša, J.; Dramićanin, M.; Marković, Z. Structural Modification of Fullerene Thin Films by Highly Charged Iron Ions. Appl. Phys. A 2007, 89, 749–754. [Google Scholar] [CrossRef]
- Zawislak, F.C.; Baptista, D.L.; Behar, M.; Fink, D.; Grande, P.L.; da Jornada, J.A.H. Damage of Ion Irradiated C60 Films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 149, 336–342. [Google Scholar] [CrossRef]
- Johnson, R.D.; Yannoni, C.S.; Vries, M.S. de C60 Solid State Rotational Dynamics and Production and EPR Spectroscopy of Fullerenes Containing Metal Atoms. Nanotechnology 1992, 3, 164–166. [Google Scholar] [CrossRef]
- Johnson, R.D.; Yannoni, C.S.; Dorn, H.C.; Salem, J.R.; Bethune, D.S. C60 Rotation in the Solid State: Dynamics of a Faceted Spherical Top. Science 1992, 255, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, T.; Scheurell, K.; Kemnitz, E.; Troyanov, S.I. Chlorination-Promoted Skeletal-Cage Transformations of C88 Fullerene by C2 Losses and a C–C Bond Rotation. Chem.-Eur. J. 2015, 21, 15138–15141. [Google Scholar] [CrossRef]
- Meletov, K.P. The Photopolymerization Rate and Activation Energy of C60 Rotations in Fullerene and Its Molecular Complexes. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 93–96. [Google Scholar] [CrossRef]
- Jaroń-Becker, A.; Becker, A.; Faisal, F.H.M. Saturated Ionization of Fullerenes in Intense Laser Fields. Phys. Rev. Lett. 2006, 96, 143006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Multimodal, PH Sensitive, and Magnetically Assisted Carrier of Doxorubicin Designed and Analyzed by Means of Computer Simulations. Langmuir 2018, 34, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Raransky, M.D.; Balazyuk, V.N.; Gunko, M.M.; Struk, A.Y. Analysis of Specific Auxetic Properties of Fullerite C60. East.-Eur. J. Enterp. Technol. 2015, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.R.F.; Brandão, J.; Cunha, M.M.; Moraes, F. Effects of Rotation in the Energy Spectrum of C60. Eur. Phys. J. D 2014, 68, 94. [Google Scholar] [CrossRef] [Green Version]
- Passaro, V.M.N.; Cuccovillo, A.; Vaiani, L.; De Carlo, M.; Campanella, C.E. Gyroscope Technology and Applications: A Review in the Industrial Perspective. Sensors 2017, 17, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usubamatov, R. Theory of Gyroscopic Effects for Rotating Objects: Gyroscopic Effects and Applications; Springer: Singapore, 2020; ISBN 9789811564741. [Google Scholar]
- Gray, R.C. Gyroscopic Principles and Applications. Nature 1944, 153, 277–278. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Ci, L.J.; Sun, L.F.; Jin, C.; Liu, L.; Ma, W.; Liu, D.; Zhao, X.; Luo, S.; Zhang, Z.; et al. Large-Scale Synthesis of Rings of Bundled Single-Walled Carbon Nanotubes by Floating Chemical Vapor Deposition. Adv. Mater. 2006, 18, 1817–1821. [Google Scholar] [CrossRef]
- Chao, D.; Jun, H.; Mingyu, T.; Deyang, Z.; Leyong, W. Design, Synthesis and Assembly of Gyroscope-like Molecules. Prog. Chem. 2010, 22, 1021–1034. [Google Scholar]
- Lang, G.M.; Shima, T.; Wang, L.; Cluff, K.J.; Skopek, K.; Hampel, F.; Blümel, J.; Gladysz, J.A. Gyroscope-Like Complexes Based on Dibridgehead Diphosphine Cages That Are Accessed by Three-Fold Intramolecular Ring Closing Metatheses and Encase Fe(CO)3, Fe(CO)2(NO)+, and Fe(CO)3(H)+ Rotators. J. Am. Chem. Soc. 2016, 138, 7649–7663. [Google Scholar] [CrossRef]
- Dmitriev, A.I.; Nikonov, A.Y.; Filippov, A.E.; Psakhie, S.G. Molecular Dynamics Study of the Evolution of Rotational Atomic Displacements in a Crystal Subjected to Shear Deformation. Phys. Mesomech. 2019, 22, 375–381. [Google Scholar] [CrossRef]
- Bubenchikov, A.M.; Bubenchikov, M.A.; Potekaev, A.I.; Libin, E.Y.; Khudobina, Y.P.; Kulagina, V.V. Penetration of Microparticles Through Composite Potential Barriers. Russ. Phys. J. 2017, 60, 140–148. [Google Scholar] [CrossRef]
- Jeong, B.-W.; Kim, H.-Y. Molecular Dynamics Simulations of the Failure Behaviors of Closed Carbon Nanotubes Fully Filled with C60 Fullerenes. Comput. Mater. Sci. 2013, 77, 7–12. [Google Scholar] [CrossRef]
- Kang, J.W.; Hwang, H.J. Molecular Dynamics Study on Oscillation Dynamics of a C60 Fullerene Encapsulated in a Vibrating Carbon-Nanotube-Resonator. Comput. Mater. Sci. 2010, 50, 790–795. [Google Scholar] [CrossRef]
- Bubenchikov, A.M.; Bubenchikov, M.A.; Lun-Fu, A.V.; Ovchinnikov, V.A. Gyroscopic Effects in Fullerite Crystal upon Deformation. Eur. Phys. J. Plus 2021, 136, 388. [Google Scholar] [CrossRef]
- Bubenchikov, M.A.; Bubenchikov, A.M.; Lun-Fu, A.V.; Ovchinnikov, V.A. Rotational Dynamics of Fullerenes in the Molecular Crystal of Fullerite. Phys. Status Solidi A 2021, 218, 2000174. [Google Scholar] [CrossRef]
- Piatek, A.; Dawid, A.; Gburski, Z. The Existence of a Plastic Phase and a Solid–Liquid Dynamical Bistability Region in Small Fullerene Cluster (C60)7: Molecular Dynamics Simulation. J. Phys. Condens. Matter 2006, 18, 8471–8480. [Google Scholar] [CrossRef] [PubMed]
- Bubenchikov, A.M.; Bubenchikov, M.A.; Mamontov, D.V.; Kaparulin, D.S.; Lun-Fu, A.V. Dynamic State of Columnar Structures Formed on the Basis of Carbon Nanotori. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 825–831. [Google Scholar] [CrossRef]
- McLure, I.A.; Ramos, J.E.; del Río, F. Accurate Effective Potentials and Virial Coefficients in Real Fluids. 1. Pure Noble Gases and Their Mixtures. J. Phys. Chem. B 1999, 103, 7019–7030. [Google Scholar] [CrossRef]
- Girifalco, L.A. Extended Mie-Grüneisen Theory Applied to C60 in the Disordered Fcc Phase. Phys. Rev. B 1995, 52, 9910–9916. [Google Scholar] [CrossRef] [PubMed]
- Rudyak, V.Y.A. Statistical Aerohydromechanics of Homogeneous and Heterogeneous Media; NSUACE: Novosibirsk, Russia, 2004; Volume 1, ISBN 5-7795-0228-5. [Google Scholar]
- Ortega, J.M.; Poole, W.G. An Introduction to Numerical Methods for Differential Equations; Pitman: Marshfield, WI, USA, 1981; ISBN 978-0-273-01686-1. [Google Scholar]
- Neumann, D.A.; Copley, J.R.D.; Cappelletti, R.L.; Kamitakahara, W.A.; Lindstrom, R.M.; Creegan, K.M.; Cox, D.M.; Romanow, W.J.; Coustel, N.; McCauley, J.P.; et al. Coherent Quasielastic Neutron Scattering Study of the Rotational Dynamics of C60 in the Orientationally Disordered Phase. Phys. Rev. Lett. 1991, 67, 3808–3811. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lun-Fu, A.V.; Bubenchikov, A.M.; Bubenchikov, M.A.; Ovchinnikov, V.A. Numerical Simulation of Interaction between Kr+ Ion and Rotating C60 Fullerene towards for Nanoarchitectonics of Fullerene Materials. Crystals 2021, 11, 1204. https://doi.org/10.3390/cryst11101204
Lun-Fu AV, Bubenchikov AM, Bubenchikov MA, Ovchinnikov VA. Numerical Simulation of Interaction between Kr+ Ion and Rotating C60 Fullerene towards for Nanoarchitectonics of Fullerene Materials. Crystals. 2021; 11(10):1204. https://doi.org/10.3390/cryst11101204
Chicago/Turabian StyleLun-Fu, Aleksandr V., Alexey M. Bubenchikov, Mikhail A. Bubenchikov, and Vyacheslav A. Ovchinnikov. 2021. "Numerical Simulation of Interaction between Kr+ Ion and Rotating C60 Fullerene towards for Nanoarchitectonics of Fullerene Materials" Crystals 11, no. 10: 1204. https://doi.org/10.3390/cryst11101204