Fabrication of Poly(pentaerythritol tetrakis (3-mercaptopropionate)/dipentaerythritol penta-/hexa-acrylate)HIPEs Macroporous Scaffold with Alpha Hydroxyapatite via Photopolymerization for Fibroblast Regeneration
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methodology
Preparation of Poly(TT/DPEHA/HA)HIPEs
2.3. Characterizations
2.3.1. Morphologic and Chemical Properties
2.3.2. Mechanical Properties
2.3.3. Cell Toxicity and Proliferation
3. Results and Discussion
3.1. Morphologic Analysis of Poly(TT/DPEHA)HIPEs
3.1.1. The Effect of Surfactant Concentration (Hypermer B246) on Poly(TT/DPEHA)HIPEs Morphology
3.1.2. Effect of Aqueous Phase on Poly(TT/DPEHA)HIPEs Morphology
3.1.3. Effect of Hydroxyapatite on Poly(TT/DPEHA)HIPEs Morphology
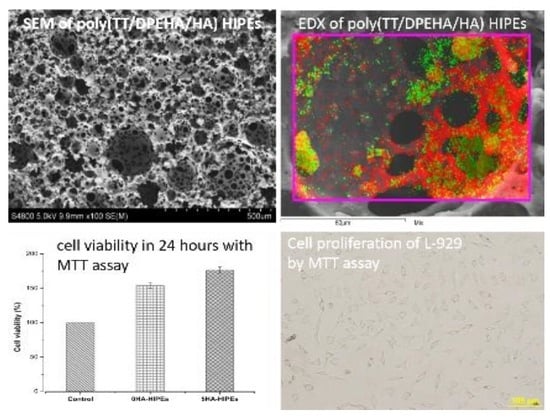
3.1.4. Distribution of HA in Poly(TT/DPEHA/HA) HIPEs
3.2. Chemical Analysis
3.2.1. FTIR-ATR Analysis
3.2.2. Thermogravimetric Analysis
3.3. Mechanical Properties Test
3.3.1. Degradation Test
3.3.2. Compression Test
3.4. Biologic Test
3.4.1. Cell Cytotoxicity by Using MTT Assay
3.4.2. Cell Proliferation by Using MTT Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Compliance of Ethics
References
- Myers, D. Surfaces, Interfaces, and Colloids; Wiley-Vch New York etc.: Hoboken, NJ, USA, 1999; Volume 358. [Google Scholar]
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320. [Google Scholar] [CrossRef]
- Cameron, N.; Sherrington, D. High internal phase emulsions (HIPEs)—Structure, properties and use in polymer preparation. In Biopolymers Liquid Crystalline Polymers Phase Emulsion; Springer: Berlin, Germany, 1996; pp. 163–214. [Google Scholar]
- Zhang, H.; Cooper, A.; Zhang, H.; Cooper, A.I. Synthesis and applications of emulsion-templated porous materials. Soft Matter 2005, 1, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.; Johnson, D.W.; Didsbury, M.P.; Murray, B.A.; Wu, J.J.; Przyborski, S.A.; Cameron, N.R. Degradable emulsion-templated scaffolds for tissue engineering from thiol–ene photopolymerisation. Soft Matter 2012, 8, 10344–10351. [Google Scholar] [CrossRef] [Green Version]
- Torstrick, F.B.; Evans, N.T.; Stevens, H.Y.; Gall, K.; Guldberg, R.E. Do surface porosity and pore size influence mechanical properties and cellular response to PEEK? Clin. Orthop. Relat. Res. 2016, 474, 2373–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Sanchez, C.; Al Mushref, F.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Pakeyangkoon, P.; Magaraphan, R.; Malakul, P.; Nithitanakul, M. Effect of soxhlet extraction and surfactant system on morphology and properties of poly (DVB) polyHIPE. In Macromolecular Symposia; Wiley-VCH Verlag: Weinheim, Germany, 2008; pp. 149–156. [Google Scholar]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Lovelady, E.; Kimmins, S.D.; Wu, J.; Cameron, N.R. Preparation of emulsion-templated porous polymers using thiol–ene and thiol–yne chemistry. Polym. Chem. 2011, 2, 559–562. [Google Scholar] [CrossRef]
- Chen, C.; Eissa, A.M.; Schiller, T.L.; Cameron, N.R. Emulsion-templated porous polymers prepared by thiol-ene and thiol-yne photopolymerisation using multifunctional acrylate and non-acrylate monomers. Polymer 2017, 126, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Harikrishna, R.; Shaikh, A.; Ponrathnam, S.; Rajan, C.; Bhongale, S. Photopolymerization of high internal phase emulsions based on 2-ethylhexyl (meth) acrylates and ethylene glycol dimethacrylate. Des. Monomers Polym. 2014, 17, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K. Porous hydroxyapatite for artificial bone applications. Sci. Technol. Adv. Mater. 2007, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Galindo, T.G.P.; Chai, Y.; Tagaya, M. Hydroxyapatite nanoparticle coating on polymer for constructing effective biointeractive interfaces. J. Nanomater. 2019, 2019, 6495239. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Molla, M.S.; Katti, K.S.; Katti, D.R. Multiscale models of degradation and healing of bone tissue engineering nanocomposite scaffolds. J. Nanomech. Micromech. 2017, 7, 04017015. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Ciapetti, G.; Cenni, E.; Pratelli, L.; Pizzoferrato, A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14, 359–364. [Google Scholar] [CrossRef]
- Wan, X.; Azhar, U.; Wang, Y.; Chen, J.; Xu, A.; Zhang, S.; Geng, B. Highly porous and chemical resistive P (TFEMA–DVB) monolith with tunable morphology for rapid oil/water separation. RSC Adv. 2018, 8, 8355–8364. [Google Scholar] [CrossRef]
- Preechawong, J.; Chindacharin, S.; Sapsrithong, P.; Nithitanakul, M. Mesoporous water adsorbent material from poly high internal phase emulsion for agriculture application. J. Appl. Polym. Sci. 2017, 134, 45509. [Google Scholar] [CrossRef]
- Barbetta, A.; Cameron, N.R. Morphology and Surface Area of Emulsion-Derived (PolyHIPE) Solid Foams Prepared with Oil-Phase Soluble Porogenic Solvents: Span 80 as Surfactant. Macromolecules 2004, 37, 3188–3201. [Google Scholar] [CrossRef]
- Lee, A.; Langford, C.R.; Rodriguez-Lorenzo, L.M.; Thissen, H.; Cameron, N.R. Bioceramic nanocomposite thiol-acrylate polyHIPE scaffolds for enhanced osteoblastic cell culture in 3D. Biomater. Sci. 2017, 5, 2035–2047. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; Haugh, M.G.; O’brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Berber, E.; Çira, F.; Mert, E.H. Preparation of porous polyester composites via emulsion templating: Investigation of the morphological, mechanical, and thermal properties. Polym. Compos. 2016, 37, 1531–1538. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149, 607–616. [Google Scholar] [CrossRef]
- Harun, N.A.; Kassim, S.; Muhammad, S.T.; Rohi, F.E.; Norzam, N.N.; Tahier, N.S.M. The effect of nonionic surfactants on emulsion polymerization of poly (methacrylic acid) nanoparticles. In Proceedings of the 3rd Electronic and Green Materials International Conference 2017, Aonang Krabi, Thailand, 29–30 April 2017; p. 020032. [Google Scholar]
- Persson, M.; Cho, S.-W.; Berglin, L.; Tuukkanen, J.; Skrifvars, M. Poly (Lactid Acid)/Hydroxipatite Composite Fibres for 3D Osteoconductive Woven Scaffolds. In Proceedings of the ECCM15 15th European Conference on Composite Materials, Venice, Italy, 24–28 June 2012. [Google Scholar]
- Chakraborty, S.; Roy, P.; Pathak, A.; Debnath, M.; Dasgupta, S.; Mukhopadhyay, R.; Bandyopadhyay, S. Composition analysis of carbon black-filled polychloroprene rubber compound by thermo-oxidative degradation of the compound. J. Elastom. Plast. 2011, 43, 499–508. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naranda, J.; Sušec, M.; Maver, U.; Gradišnik, L.; Gorenjak, M.; Vukasović, A.; Ivković, A.; Rupnik, M.S.; Vogrin, M.; Krajnc, P. Polyester type polyHIPE scaffolds with an interconnected porous structure for cartilage regeneration. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 906–919. [Google Scholar]
- Wang, Y.; Wan, X.; He, J.; Azhar, U.; Chen, H.; Zhao, J.; Pang, A.-m.; Geng, B. A one-step fabrication and modification of HIPE-templated fluoro-porous polymer using PEG-b-PHFBMA macrosurfactant. J. Mater. Sci. 2020, 55, 4970–4986. [Google Scholar] [CrossRef]
- Wang, A.-j.; Paterson, T.; Owen, R.; Sherborne, C.; Dugan, J.; Li, J.-M.; Claeyssens, F. Photocurable high internal phase emulsions (HIPEs) containing hydroxyapatite for additive manufacture of tissue engineering scaffolds with multi-scale porosity. Mater. Sci. Eng. C 2016, 67, 51–58. [Google Scholar] [CrossRef]
- Müller, K.H.; Motskin, M.; Philpott, A.J.; Routh, A.F.; Shanahan, C.M.; Duer, M.J.; Skepper, J.N. The effect of particle agglomeration on the formation of a surface-connected compartment induced by hydroxyapatite nanoparticles in human monocyte-derived macrophages. Biomaterials 2014, 35, 1074–1088. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Wang, Y.; Zhuang, G.; Zhong, X.; Wei, Z.; Yao, Z.; Wang, J.-G. Micromechanical simulation of the pore size effect on the structural stability of brittle porous materials with bicontinuous morphology. Phys. Chem. Chem. Phys. 2019, 21, 12895–12904. [Google Scholar] [CrossRef]
- Dhand, C.; Ong, S.T.; Dwivedi, N.; Diaz, S.M.; Venugopal, J.R.; Navaneethan, B.; Fazil, M.H.; Liu, S.; Seitz, V.; Wintermantel, E. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials 2016, 104, 323–338. [Google Scholar] [CrossRef]
- Guo, X.; Gough, J.E.; Xiao, P.; Liu, J.; Shen, Z. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 82, 1022–1032. [Google Scholar] [CrossRef] [PubMed]












| Sample | Td Onset (°C) | Mass Loss (%) |
|---|---|---|
| PolyHIPEs | 330 | 94.21 |
| 5-HA HIPEs | 325 | 90.31 |
| 10-HA HIPEs | 322 | 84.22 |
| 15-HA HIPEs | 322 | 77.15 |
| Sample | Stress at Maximum Load (MPa) | Compressive Modulus (MPa) |
|---|---|---|
| PolyHIPEs | 1.05 ± 0.22 | 5.82 ± 1.06 |
| 5-HA HIPEs | 1.24 ± 0.11 | 6.17 ± 0.74 |
| 10-HA HIPEs | 0.90 ± 0.17 | 5.07 ± 1.00 |
| 15-HA HIPEs | 0.60 ± 0.14 | 4.84 ± 0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, M.I.; Kwangsawart, N.; Preechawong, J.; Nithitanakul, M.; Sapsrithong, P. Fabrication of Poly(pentaerythritol tetrakis (3-mercaptopropionate)/dipentaerythritol penta-/hexa-acrylate)HIPEs Macroporous Scaffold with Alpha Hydroxyapatite via Photopolymerization for Fibroblast Regeneration. Crystals 2020, 10, 746. https://doi.org/10.3390/cryst10090746
Azman MI, Kwangsawart N, Preechawong J, Nithitanakul M, Sapsrithong P. Fabrication of Poly(pentaerythritol tetrakis (3-mercaptopropionate)/dipentaerythritol penta-/hexa-acrylate)HIPEs Macroporous Scaffold with Alpha Hydroxyapatite via Photopolymerization for Fibroblast Regeneration. Crystals. 2020; 10(9):746. https://doi.org/10.3390/cryst10090746
Chicago/Turabian StyleAzman, Muhammad Imran, Nunthawan Kwangsawart, Jitima Preechawong, Manit Nithitanakul, and Pornsri Sapsrithong. 2020. "Fabrication of Poly(pentaerythritol tetrakis (3-mercaptopropionate)/dipentaerythritol penta-/hexa-acrylate)HIPEs Macroporous Scaffold with Alpha Hydroxyapatite via Photopolymerization for Fibroblast Regeneration" Crystals 10, no. 9: 746. https://doi.org/10.3390/cryst10090746