Synthesis, Crystal Structures and Catalytic Activities of Two Copper Coordination Compounds Bearing an N,N’-Dibenzylethylenediamine Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
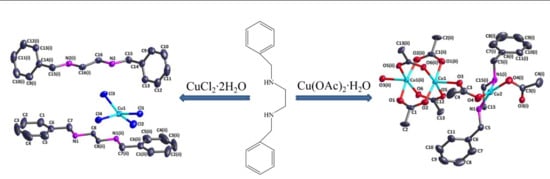
2.2. Synthesis of [Cu3L(CH3COO)6]n (1)
2.3. Synthesis of [(CuCl4)∙(C6H5CH2NH2CH2)2] (2)
2.4. X-ray Crystallography
2.5. Decomposition Reaction of Hydrogen Peroxide
3. Results and Discussion
3.1. Structure Description of [Cu3L(CH3COO)6]n
3.2. Structure Description of [(CuCl4)∙(C6H5CH2NH2CH2)2]
3.3. PXRD Analysis
3.4. Catalytic Activities of the Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Sorolla, M.; Wang, X.; Jacobson, A.J.; Wang, H.; Pillai, A.K. Synthesis, crystal structures and in vitro anticancer activities of two copper(II) coordination compounds. Transit. Met. Chem. 2018, 44, 237–245. [Google Scholar] [CrossRef]
- Călinescu, M.; Fiastru, M.; Bala, D.; Mihailciuc, C.; Negreanu-Pîrjol, T.; Jurcă, B. Synthesis, characterization, electrochemical behavior and antioxidant activity of new copper(II) coordination compounds with curcumin derivatives. J. Saudi Chem. Soc. 2019, 23, 817–827. [Google Scholar] [CrossRef]
- Sran, B.S.; Sharma, S.; Hundal, G. Self-assembly and supramolecular isomerism in copper (II) coordination compounds of pyridine-4-carboxamide based ligand. Inorg. Chim. Acta 2018, 486, 74–82. [Google Scholar] [CrossRef]
- He, G.; Li, J.; Wang, Z.; Liu, C. Synthesis of a fluorogenic probe for thiols based on a coumarin schiff base copper complex and its use for the detection of glutathione. Tetrahedron 2017, 73, 272–277. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Wang, L.; Qu, S.; Liu, K. Geometric relaxation in a copper complex and its limitation by polymer immobilization: Structure, characterization and photophysical analysis. J. Lumin. 2017, 192, 269–276. [Google Scholar] [CrossRef]
- Muthukumar, P.; Pannipara, M.; Al-Sehemi, A.G.; Moon, D.; Anthony, S.P. Polymorphs of a copper coordination compound: Interlinking active sites enhance the electrocatalytic activity of the coordination polymer compared to the coordination complex. Crystengcomm 2020, 22, 425–429. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Y.; Gu, J.; Fernandes, T.A.; Kirillova, M.V.; Kirillov, A.M. New copper(II) coordination compounds assembled from multifunctional pyridine-carboxylate blocks: Synthesis, structures, and catalytic activity in cycloalkane oxidation. Molecules 2018, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Amini, M.; Nikkhoo, M.; Tekantappeh, S.B.; Farniab, S.M.F.; Mahmoudi, G.; Büyükgüngör, O. Synthesis, characterization and catalytic properties of a copper complex containing decavanadate nanocluster, Na2[Cu(H2O)6]2{V10O28·4H2O}. Inorg. Chem. Commun. 2017, 77, 72–76. [Google Scholar] [CrossRef]
- Sindhuja, D.; Vasanthakumar, P.; Bhuvanesh, N.; Karvembu, R. Catalytic assessment of copper(I) complexes and a polymer analog towards the one-pot synthesis of imines and quinoxalines. Eur. J. Inorg. Chem. 2019, 2019, 3940–3941. [Google Scholar] [CrossRef] [Green Version]
- Nesterova, O.V.; Bondarenko, O.E.; Pombeiro, A.J.L.; Nesterov, D.S. Phenoxazinone synthase-like catalytic activity of novel mono- and tetranuclear copper(II) complexes with 2-benzylaminoethanol. Dalton Trans. 2020, 49, 4710–4724. [Google Scholar] [CrossRef]
- Yang, Y.Y.; He, M.Q.; Li, M.X.; Huang, Y.Q.; Chi, T.; Wang, Z.-X. Ferrimagnetic copper-carboxyphosphinate compounds for catalytic degradation of methylene blue. Inorg. Chem. Commun. 2018, 94, 5–9. [Google Scholar] [CrossRef]
- Narulkar, D.D.; Patil, A.R.; Naik, C.C.; Dhuri, S.N. Synthesis, characterization, cis-ligand substitution and catalytic alkane hydroxylation by mononuclear nickel(II) complexes stabilized with tetradentate tripodal ligands. Inorg. Chim. Acta. 2015, 427, 248–258. [Google Scholar] [CrossRef]
- Naeimi, H.; Moradian, M. Encapsulation of copper(I)-Schiff base complex in NaY nanoporosity: An efficient and reusable catalyst in the synthesis of propargylamines via A3-coupling (aldehyde-amine-alkyne) reactions. Appl. Catal. A 2013, 467, 400–406. [Google Scholar] [CrossRef]
- Myznikov, L.V.; Fisher, A.I.; Dmitrieva, U.N.; Artamonova, T.V.; Zevatskii, Y.E. Novel mixed complexes of copper(II) and ethylenediamine: Synthesis, crystal structure, and catalytic activity in the cross-coupling reaction of 1-phenyl-5H-tetrazole-5-thiol and iodobenzene. Russ. J. Gen. Chem. 2018, 88, 495–499. [Google Scholar] [CrossRef]
- Wu, X.Y.; Ren, Z.G.; Lang, J.P. Ni(II) tetraphosphine complexes as catalysts/initiators in the ring opening polymerization of ε-caprolactone. Dalton Trans. 2013, 43, 1716–1723. [Google Scholar] [CrossRef]
- Rajković, S.; Ašanin, D.P.; Živković, M.D.; Djuran, M.I. Synthesis of different pyrazine-bridged platinum(II) complexes and 1H NMR study of their catalytic abilities in the hydrolysis of the N-acetylated l-methionylglycine. Polyhedron 2013, 65, 42–47. [Google Scholar] [CrossRef]
- Chinnaraja, E.; Arunachalam, R.; Choudhary, M.K.; Kureshy, R.I. Binuclear Cu(II) chiral complexes: Synthesis, characterization and application in enantioselective nitroaldol (Henry) reaction. Appl. Organomet. Chem. 2016, 30, 95–101. [Google Scholar] [CrossRef]
- Mutti, F.G.; Zoppellaro, G.; Gullotti, M.; Santagostini, L. Biomimetic modelling of copper enzymes: Synthesis, characterization, EPR analysis and enantioselective catalytic oxidations by a new chiral trinuclear copper(II) complex. Eur. J. Inorg. Chem. 2009, 4, 554–566. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, D.; Wu, L.; Feng, W.; Zhang, X.; Wu, J.; Fan, D.; Lü, X.; Lu, R.; Shi, Q. A trinuclear [Zn3(L)2(OAc)2] complex based on the asymmetrical bis-Schiff-base ligand H2L for ring-opening copolymerization of CHO and MA. Inorg. Chem. Commun. 2013, 37, 182–185. [Google Scholar] [CrossRef]
- Sreedaran, S.; Bharathi, K.S.; Rahiman, A.K.; Jagadish, L.; Kaviyarasan, V.; Narayanan, V. Synthesis, electrochemical, magnetic, catalytic and antimicrobial studies of N-functionalized cyclam based trinuclear copper(II) and nickel(II) complexes. J. Incl. Phenom. Macrocyclic Chem. 2010, 66, 297–306. [Google Scholar] [CrossRef]
- Shi, F.; Chen, Y.; Sun, L.; Zhang, L.; Hu, J. Hydroxylation of phenol catalyzed by different forms of Cu-alginate with hydrogen peroxide as an oxidant. Catal. Commun. 2012, 25, 102–105. [Google Scholar] [CrossRef]
- Penha, E.S.D.; Cabral, E.L.; Gama, T.S.D.; Oliveira, C.A.D. Use of 35% hydrogen peroxide in tooth bleaching in different clinical time intervals: How long does sensitivity last, and at what times is it more exacerbated. Biochem. J. 2018, 34, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Duan, C.; Sheng, Q.; Zheng, J. Preparation of Ag@zeolitic imidazolate framework-67 at room temperature for electrochemical sensing of hydrogen peroxide. Analyst 2019, 144, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Cleaner Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Protective effect of lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2). Pharm. Biol. 2016, 54, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radko, M.; Kowalczyk, A.; Mikrut, P.; Witkowski, S.; Mozgawa, W.; Macyk, W.; Chmielarz, L. Catalytic and photocatalytic oxidation of diphenyl sulphide to diphenyl sulfoxide over titanium dioxide doped with vanadium, zinc, and tin. RSC Adv. 2020, 10, 4023–4031. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Zhang, A.; Liu, H.; Sun, J.; Liu, J.; Meng, F. Pollution characteristics and chemical behaviors of atmospheric hydrogen peroxide. Res. Environ. Sci. 2016, 29, 334–342. [Google Scholar] [CrossRef]
- Han, Q.; Ni, P.; Liu, Z.; Dong, X.; Wang, Y.; Li, Z.; Liu, Z. Enhanced hydrogen peroxide sensing by incorporating manganese dioxide nanowire with silver nanoparticles. Electrochem. Commun. 2014, 38, 110–113. [Google Scholar] [CrossRef]
- Salem, I.A.; El-Sheikh, M.; Zaki, A.B. ChemInform abstract: Kinetics and mechanisms of decomposition reaction of hydrogen peroxide in presence of metal complexes. Int. J. Chem. Kinet. 2000, 32, 643–666. [Google Scholar] [CrossRef]
- Deng, H.H.; Wu, G.W.; He, D.; Peng, H.P.; Liu, A.L.; Xia, X.H.; Chen, W. Fenton reaction-mediated fluorescence quenching of N-acetyl-l-cysteine-protected gold nanoclusters: Analytical applications of hydrogen peroxide, glucose, and catalase detection. Analyst 2015, 140, 7650–7656. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, F.; Kong, G.; Cai, S.; Wang, W.; Du, J.; Wu, L. Fast decomposition of hydrogen peroxide by Zeolitic imidazolate framework-67 crystals. Mater. Lett. 2019, 239, 94–97. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Yin, J. Study of acetone ketal copper ethylenediamine synthetic and catalytic properties. J. Weifang Univ. 2015, 15, 32–34. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, C.; Zheng, Y.; Zhu, H. Two dinuclear copper(II) coordination polymers constructed from m-hydroxybenzoic acid and N-donor ligands: Synthesis, crystal structures, and magnetic properties. Transit. Met. Chem. 2016, 41, 87–96. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Zhang, L.; Jiang, K.; Zhang, L. Two coordination compounds bearing bis(2-dimethylaminoethyl) ether: Syntheses, crystal structures and catalytic application to the Henry reaction. Chem. Res. Chin. Univ. 2018, 34, 358–362. [Google Scholar] [CrossRef]
- Zhang, L.W.; Li, X.Y.; Kang, Q.P.; Liu, L.Z. Structures and fluorescent and magnetic behaviors of newly synthesized Ni(II) and Cu(II) coordination compounds. Crystals 2018, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Karthik, K.; Qadir, A.M. Synthesis and crystal structure of a new binuclear copper(II) carboxylate complex as a precursor for copper(II) oxide nanoparticles. J. Struct. Chem. 2019, 60, 1126–1132. [Google Scholar] [CrossRef]
- Yan, X.; Wang, B.; Ma, H.; Gao, F. Synthesis and catalytic properties of transition metal complexes with unsymmetrical Schiff base. Chem. Res. J. Appl. 2006, 18, 621–624. [Google Scholar] [CrossRef]





| Compound | (1) | (2) |
|---|---|---|
| Formula | C28H38Cu3N2O12 | C16H22Cl4CuN2 |
| Formula weight | 785.22 | 447.69 |
| Temperature | 296(2) | 296(2) |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | Pī |
| a (Å) | 15.903(13) | 6.5935(9) |
| b (Å) | 16.403(15) | 11.9953(18) |
| c (Å) | 15.679(12) | 12.4736(17) |
| α | 90.00 | 84.199(4) |
| β | 96.06(2) | 88.408(4) |
| γ | 90.00 | 77.972(4) |
| Volume (Å3) | 4067(6) | 959.9(2) |
| Z | 4 | 2 |
| Density (calculated)/(g·cm−3) | 1.283 | 1.549 |
| μ (mm−1) | 1.605 | 1.694 |
| F (000) | 1612 | 458 |
| Reflections collected | 13,134 | 6888 |
| Unique reflections (Rint) | 4648 (0.064) | 3346 (0.040) |
| Gof | 1.026 | 1.057 |
| Final R indices [I > 2σ(I)] | R1 = 0.0931, wR2 = 0.2708 | R1 = 0.0300, wR2 = 0.0770 |
| R indices (all data) | R1 = 0.1098, wR2 = 0.2899 | R1 = 0.0322, wR2 = 0.0786 |
| Largest diff. peak and hole (e Å−3) | 0.96 and −1.13 | 0.30 and −0.49 |
| Entry | Catalyst | Catalyst Loading (mmol) | Decomposition Percent (%) |
|---|---|---|---|
| 1 | compound (1) | 0.4 | 39 |
| 2 | 0.7 | 78 | |
| 3 | 1.0 | 94 | |
| 4 | 1.3 | 95 | |
| 5 | 1.6 | 95 | |
| 6 | compound (2) | 0.4 | 44 |
| 7 | 0.7 | 82 | |
| 8 | 1.0 | 99 | |
| 9 | 1.3 | 99 | |
| 10 | 1.6 | 99 | |
| 11 | Ligand | 1.0 | 0 |
| 12 | Cu(OAc)2·H2O | 1.0 | 77 |
| 13 | CuCl2·2H2O | 1.0 | 84 |
| 14 | (NH4)2CuCl4·2H2O | 1.0 | 81 |
| Entry | Catalyst | pH | Decomposition Percent (%) |
|---|---|---|---|
| 1 | compound (1) | 5 | 44 |
| 2 | 6 | 65 | |
| 3 | 7 | 81 | |
| 4 | 8 | 94 | |
| 5 | 9 | 88 | |
| 6 | 10 | 79 | |
| 7 | compound (2) | 5 | 51 |
| 8 | 6 | 69 | |
| 9 | 7 | 86 | |
| 10 | 8 | 99 | |
| 11 | 9 | 80 | |
| 12 | 10 | 61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, W.; Cai, G. Synthesis, Crystal Structures and Catalytic Activities of Two Copper Coordination Compounds Bearing an N,N’-Dibenzylethylenediamine Ligand. Crystals 2020, 10, 528. https://doi.org/10.3390/cryst10060528
Liu C, Zhang W, Cai G. Synthesis, Crystal Structures and Catalytic Activities of Two Copper Coordination Compounds Bearing an N,N’-Dibenzylethylenediamine Ligand. Crystals. 2020; 10(6):528. https://doi.org/10.3390/cryst10060528
Chicago/Turabian StyleLiu, Chao, Weiwei Zhang, and Gaigai Cai. 2020. "Synthesis, Crystal Structures and Catalytic Activities of Two Copper Coordination Compounds Bearing an N,N’-Dibenzylethylenediamine Ligand" Crystals 10, no. 6: 528. https://doi.org/10.3390/cryst10060528