Ionic Liquids/Deep Eutectic Solvents-Based Hybrid Solvents for CO2 Capture
Abstract
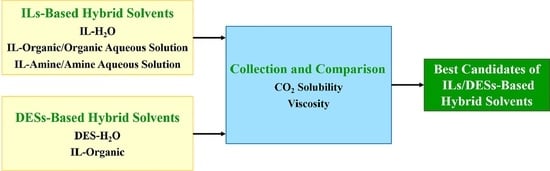
:1. Introduction
2. ILs-Based Hybrid Solvents
2.1. CO2 Solubility and Henry’s Constant
2.1.1. IL–H2O
2.1.2. IL–Organic/Organic Aqueous Solution
2.1.3. IL–Amine/Amine Aqueous Solution
2.2. Viscosity
2.2.1. IL–H2O
2.2.2. IL–Organic/Organic Aqueous Solution
2.2.3. IL–Amine
| IL-Based Hybrid Solvents | T/K | P/MPa | CO2 Solubility (mol CO2/kg Absorbent) | CO2 Solubility (mol CO2/mol IL) | Ref. |
|---|---|---|---|---|---|
| IL–H2O | |||||
| [DMAPAH][Formate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) + H2O (20, 33, 42, 49, 59, 75 wt %) | 298.15 | 0.1 | 0.41–0.14, 2.73–0.77, 4.23–1.41, 4.61–1.41 | [30] | |
| [P4442][Suc] + H2O (3.3, 8.8, 17.6 wt %) | 293 | 0.1 | 1.9, 1.24, 0.81 | [31] | |
| [TMGH][Im] + H2O (1, 2, 3, 5, 7, 10, 15, 20, 25 wt %) | 313.15 | 0.1 | 3.39–4.23 | [12] | |
| [P4444][HCOO] + H2O (29, 32, 38, 50, 66, 70, 73, 79, 86, 91 mol%) | 248.75–333.15 | 0.1 | 0.01–1 | [32] | |
| [BMMIM][Im] + H2O (67, 91, 99, 99.9 mol%) | / | 2 | 1.29, 2.30, 0.83, 0.55 | 0, 0, 0.36, 8.15 | [33] |
| [BMMIM][Im] + H2O (99 mol%) | / | 1 | 0.45 | [33] | |
| [BMMIM][Ac] + H2O (67, 99.9 mol%) | / | 2 | 0, 6.95 | [33] | |
| [P4443][Gly] + H2O (59.9, 80.1, 90, 95 wt %) | 278.09–348.10 | 0.103–7.53 | 0.091–2.44 | 0.23–12.13 | [34] |
| [HMIM][Tf2N] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 0.30–0.13 | [35] | |
| [HMIM][FAP] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 0.48–0.14 | [35] | |
| [BMIM][Ac] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–323 | 2 | 0.46–0.32 | [35] | |
| [Cho][Gly] + H2O (70 wt %) | 308.15–338.15 | 0.0046–0.68 | 0.36–1.24 | 0.21–0.74 | [17] |
| IL–organic/organic aqueous solution | |||||
| [TETAH][Lys] + ethanol + H2O (H2O:ethanol = 8:2, 6:4, 5:5, 4:6, 3:7, 2:8 v:v) | 303 | 0.1 | 2.45–1.53 | [36] | |
| [AMIM][Tf2N] + methanol (20, 50, 80 wt %) | 313.2–353.2 | 0.98–6.19 | 2.15–3.89 | [39] | |
| [Cho][Gly] (30 wt %) + PEG200 (10 wt%) + H2O (60 wt %) | 308.15–338.15 | 0.0054–0.68 | 0.37–1.22 | 0.22–0.72 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (20 wt%) + H2O (50 wt %) | 308.15–338.15 | 0.0039–0.68 | 0.41–1.21 | 0.24–0.72 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (30 wt%) + H2O (40 wt %) | 308.15–338.15 | 0.0065–0.68 | 0.41–1.23 | 0.24–0.73 | [17] |
| [Cho][Pro] + PEG200 (50, 67, 75 wt %) | 308.15–353.15 | 0.0041–0.11 | 0.099–0.61 | [40] | |
| [P4444][Gly] + PEG400 (70 wt %) | 333.15–413.15 | 0.088–1.7 | 0.19–1.58 | 0.19–1.23 | [41] |
| [P4444][Ala] + PEG400 (70 wt %) | 333.15–413.15 | 0.093–1.7 | 0.11–1.57 | 0.11–1.26 | [41] |
| [P4444][Pro] + PEG400 (70 wt %) | 333.15–413.15 | 0.096–1.71 | 0.15–1.61 | 0.17–1.41 | [41] |
| [DETAH][Br] + PEG200 (80 wt %) | 293.15 | 0.1 | 1.18 | [42] | |
| [DETAH][Br] + PEG300 (80 wt %) | 293.15 | 0.1 | 0.87 | [42] | |
| [DETAH][Br] + PEG400 (80 wt %) | 293.15 | 0.1 | 0.32 | [42] | |
| [DETAH][BF4] + PEG200 (80 wt %) | 293.15 | 0.1 | 0.65 | [42] | |
| [DETAH][Br] + PEG200 + H2O (1.3, 4.7 wt %) | 293.15 | 0.1 | 1.05, 1.18 | [42] | |
| [N1114][Tf2N] + PEO1000 (10.44, 28.27, 50.22, 75.31 mol%) | 323, 343 | 0.02–0.5 | 0.0057–1.16 | [43] | |
| [BMIM][BF4] + TEG (20, 50, 80 mol%) | 273.15–353.15 | 0.42–3.55 | 0.051–1.72 | [44] | |
| [BMIM][BF4] (56 mol%) + TEG (14 mol%) + H2O (30 mol%) | 293.15–333.15 | 0.38–4.17 | 0.051–0.96 | [44] | |
| [BMIM][BF4] (35 mol%) + TEG (35 mol%) + H2O(30 mol%) | 293.15–333.15 | 0.57–4.37 | 0.079–1.4 | [44] | |
| [BMIM][BF4] (14 mol%) + TEG (56 mol%) + H2O(30 mol%) | 293.15–333.15 | 0.64–4.46 | 0.15–1.84 | [44] | |
| [P66614][3-Triz] + TG (30 mol%) | 313.15–353.6 | 0.037–2.75 | 0.06–1.55 | [18] | |
| [P66614][4-Triz] + TG (30 mol%) | 313.15–353.6 | 0.07–3.03 | 0.075–2.23 | [18] | |
| [TEPAH][2-MI] + NPA + EG | 303.15 | 0.1 | 1.72 | [45] | |
| IL-amine/amine aqueous solution | |||||
| [BMPyrr][DCA] (5 wt %) + DEA (35 wt%) + H2O (60 wt %) | 333.15 | 0.5–0.7 | 0.49–0.71 | [52] | |
| [BMPyrr][DCA] (10 wt %) + DEA (30wt%) + H2O (60 wt %) | 333.15 | 0.5–0.7 | 0.58–0.81 | [52] | |
| [DMAPAH][Formate] (1.0:1, 2.5:1) + MEA | 298.15 | 0.1 | 3.82, 4.52 | [30] | |
| [DMEDAH][Formate] (1.0:1, 2.5:1) + MEA | 298.15 | 0.1 | 3.52, 4.07 | [30] | |
| [DMAPAH][Formate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) + MEA (30 wt %) | 298.15 | 0.1 | 2.85, 4.57, 6.24, 5.89 | [30] | |
| [DMAPAH][Octanoate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) + MEA (30 wt %) | 298.15 | 0.1 | 2.29, 2.69, 3.30, 3.65 | [30] | |
| [DMAPAH][Ac] + MDEA (20, 33, 43, 50, 60, 67, 80 mol%) | 308.15 | 0.0–3.0 | 0–3.13 | [50] | |
| [DMAPAH][Ac] + MDEA (50 mol%) | 298.15–328.15 | 0.0–3.0 | 0–3.21 | [50] | |
| [N1111][Lys] + DMEE (95, 90, 80, 60, 40 wt %) | 303 | 0.1 | 0.28–1.69 | 1.22–0.61 | [51] |
| [N1111][Lys] + DMEE (80 wt %) | 313, 323 | 0.1 | 0.76, 0.72 | [51] | |
| IL-Based Hybrid Solvents | T (K) | Henry’s Constant (MPa) | Ref. |
|---|---|---|---|
| IL–H2O | |||
| [P4443][Gly] + H2O (59.9, 80.1, 90%, 95 wt %) | 278.14–348.05 | 2.8–5.05, 1.53–3.3, 0.87–2.81, 0.35–1.03 | [34] |
| [Cho][Gly] + H2O (70 wt %) | 308.15–338.15 | 40.56–58 | [17] |
| IL–organic | |||
| [Cho][Gly] (30 wt %) + PEG200 (10 wt %) + H2O (60 wt %) | 308.15–338.15 | 36.6–52.2 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (20 wt %) + H2O (50 wt %) | 308.15–338.15 | 33–47.03 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (30 wt %) + H2O (40 wt %) | 308.15–338.15 | 31.49–46.79 | [17] |
| [BMIM][BF4] + TEG (20, 50, 80 mol%) | 273.15–353.15 | 4.84–20.02, 5.65–22, 6.48–27.63 | [44] |
| IL-Based Hybrid Solvents | T/K | P/MPa | Viscosity (mPa·s) | Ref. |
|---|---|---|---|---|
| IL-H2O | ||||
| [DEA][Bu] + H2O (98.78, 95.49, 90.01, 85.7, 79.49, 69.36, 51.21, 33.16, 2.6 mol%) | 283.15–343.15 | 0.1 | 2.24–0.59, 6.91–1.10, 19.26–2.25, 32.03–3.24, 56.72–4.81, 106.48–7.09, 158.58–9.28, 158.29–9.64, 130.18–8.70 | [14] |
| [P4444][HCOO] + H2O (25, 50, 60, 74, 80, 91 mol%) | 298.15 | 0.1 | 356, 146, 97, 48, 35.3, 14.4 | [32] |
| [HMIM][Tf2N] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 69.18–18.6, 66.54–18.2, 63.71–17.54, 55.95–15.7, 45.05–13.01 | [35] |
| [HMIM][FAP] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 88.09–20.71, 84.54–20.70, 80.76–20.66, 70.4–20.6, 56.01–20.47 | [35] |
| [BMIM][Ac] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 389–45.30, 225.86–30.04, 135.5–20.45, 44.08–8.78, 14.34–3.77 | [35] |
| IL–organic/organic aqueous solution | ||||
| [TETAH][Lys] + ethanol + H2O (H2O:ethanol = 8:2, 6:4, 5:5, 4:6, 3:7, 2:8 v:v) | 303 | 0.1 | 2.57, 3.00, 3.50, 3.81, 3.74, 3.51 | [36] |
| [Cho][Gly] + PEG200 (70 wt %) | 308.15–338.15 | 0.1 | 101.3–28.6 | [17] |
| [Cho][Gly]/H2O + PEG200 (30 wt %) | 308.15, 338.15 | 0.1 | 7.96, 3.43 | [17] |
| [P4444][Gly] + PEG400 (70 wt %) | 298.15–393.15 | 0.1 | 180.47–8.96 | [41] |
| [P4444][Ala] + PEG400 (70 wt %) | 298.15–393.15 | 0.1 | 216.64–9.13 | [41] |
| [P4444][Pro] + PEG400 (70 wt %) | 298.15–393.15 | 0.1 | 481–14.1 | [41] |
| [DETAH][Br] + PEG200 (80 mol%) | 293.15 | 0.1 | 71.7 | [42] |
| [N1114][Tf2N] + PEO222 (26.23, 50.07, 75.06 mol%) | 293.15–353.15 | 0.1 | 135.22–2.57 | [43] |
| [N1114][Tf2N] + PEO500 (20.90, 44.22, 70.40 mol%) | 293.15–353.15 | 0.1 | 135.22–7.51 | [43] |
| [N1114][Tf2N] + PEO1000 (25.04, 50.22, 75.31 mol%) | 318.15–353.15 | 0.1 | 51.72–17.22 | [43] |
| [P66614][4-NO2imid] + TG (18.9, 40.4 mol%) | 278.15–323.15 | 0.1 | 1503–57 | [18] |
| [P66614][4,5-CNimid] + TG (20.1, 40.1 mol%) | 278.15–323.15 | 0.1 | 1051–63 | [18] |
| [P66614][Tf2N] + TG (19.1, 36.6, 55.9, 66.4 mol%) | 278.15–323.15 | 0.1 | 589–51 | [18] |
| [P66614][2-CH3,5-NO2imid] + TG (15.1, 39.9 mol%) | 278.15–323.15 | 0.1 | 2535–57 | [18] |
| [P66614][DCA] + TG (9.9, 20.3, 29.9, 50.1, 65.2 mol%) | 278.15–323.15 | 0.1 | 1130–54 | [18] |
| [HMIM][Tf2N] + TG (9.9, 19, 27.6, 41.3, 54.4 mol%) | 278.15–293.15 | 0.1 | 171–50 | [18] |
| [P66614][BrBnIm] + TG (10.2, 13.8, 21.8, 34.6, 47.8, 63.8 mol%) | 278.15–323.15 | 0.1 | 4400–51 | [18] |
| [P66614][Ac] + TG (10.3, 20.7, 29.9, 40, 49.9 mol%) | 278.15–323.15 | 0.1 | 1130–57 | [18] |
| [HMMIM][Tf2N] + TG (10.2, 20.5, 30.6, 39.9, 49.9, 59.9 mol%) | 278.15–323.15 | 0.1 | 370–48 | [18] |
| [P66614][4-Triz] + TG (4.6, 12.6, 19.6, 24.9, 30.6, 39, 48.4, 58.2 mol%) | 278.15–323.15 | 0.1 | 3290–53 | [18] |
| [P66614][3-Triz] + TG (8.3, 12.2, 20.7, 31, 39.8, 50.1, 70 mol%) | 278.15–323.15 | 0.1 | 1180–51 | [18] |
| [P44412][3-Triz] + TG (6.2, 12.3, 15.5, 21.4, 30.5, 39.5, 49.5, 59.6 mol%) | 278.15–323.15 | 0.1 | 1980–57 | [18] |
| [P2228][4-NO2pyra] + TG (5.1, 10.2, 19.9, 30, 40.4, 50.3 mol%) | 278.15–323.15 | 0.1 | 1010–55 | [18] |
| [P2228][4-NO2imid] + TG (10, 20, 30.1, 40.1, 50.1 mol%) | 278.15–323.15 | 0.1 | 700–55 | [18] |
| [P2228][2-CH3,5-NO2imid] + TG (3.6, 6.7, 11.5, 23.3, 30, 39.9, 50, 59.8 mol%) | 278.15–323.15 | 0.1 | 2730–51 | [18] |
| [mm(butene)im][4-NO2pyra] + TG (4.8, 10, 19.9, 29.9, 39.9, 50, 60 mol%) | 278.15–323.15 | 0.1 | 4300–51 | [18] |
| [P2224][2-CH3,5-NO2imid] + TG (4.8, 10.2, 20.1, 29.9, 40.1, 50.1 mol%) | 278.15–323.15 | 0.1 | 1540–57 | [18] |
| [pmmim][4-NO2pyra] + TG (5, 10, 20.1, 30.2, 39.9, 49.9, 60 mol%) | 278.15–323.15 | 0.1 | 5420–59 | [18] |
| [TEPAH][2-MI] + NPA + EG | 303.15 | 0.1 | 3.66 | [45] |
| IL–amine | ||||
| [N1111][Lys] + DMEE (95, 90, 80, 60, 40 wt %) | 303–333 | 0.1 | 12.09–4.88, 20.70–6.98, 30.00–9.30, 80.46–20, 101.86–25.35 | [51] |
| [BMIM][BF4] + DETA (94.9, 80.14, 70.02, 60.05, 50.08, 40.95, 30.26, 19.88, 10.52, 5.04 mol%) | 298.15–333.15 | 0.1 | 6.71–2.68, 12.1–4.89, 17.35–6.69, 23.66–8.72, 31.54–11.19, 40.07–13.88, 52.59–17.57, 67.10–21.30, 82.58–24.18, 91.94–24.54 | [53] |
3. DESs-Based Hybrid Solvents
3.1. CO2 Solubility
3.1.1. DES–H2O
3.1.2. DES–Organic
3.2. Viscosity
4. Comparison of CO2 Solubility and Viscosity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645. [Google Scholar] [CrossRef] [Green Version]
- Oko, E.; Zacchello, B.; Wang, M.H.; Fethi, A. Process analysis and economic evaluation of mixed aqueous ionic liquid and monoethanolamine (MEA) solvent for CO2 capture from a coke oven plant. Greenh. Gases 2018, 8, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Kothandaraman, A.; Nord, L.; Bolland, O.; Herzog, H.J.; McRae, G.J. Comparison of solvents for post-combustion capture of CO2 by chemical absorption. Energy Procedia 2009, 1, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.; Wang, M.; Stephenson, P.; Obi, O. Demonstrating full-scale post-combustion CO2 capture for coal-fired power plants through dynamic modelling and simulation. Fuel 2012, 101, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Zeng, S.J.; Zhang, X.P.; Bai, L.; Zhang, X.C.; Wang, H.; Wang, J.J.; Bao, D.; Li, M.D.; Liu, X.Y.; Zhang, S.J. Ionic-liquid-based CO2 capture systems: Structure, interaction and process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Cui, G.K.; Wang, H.Y.; Li, Z.Y.; Wang, J.J. Tuning ionic liquids with imide-based anions for highly efficient CO2 capture through enhanced cooperations. J. CO2 Util. 2018, 28, 299–305. [Google Scholar] [CrossRef]
- Sarmad, S.; Xie, Y.J.; Mikkola, J.-P.; Ji, X.Y. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity. New J. Chem. 2017, 41, 290–301. [Google Scholar] [CrossRef]
- Liu, H.J.; Pan, Y.; Yao, H.; Zhang, Y. Enhancement of carbon dioxide mass transfer rate by (ionic liquid)-in-water emulsion. Adv. Mater. Res. 2014, 881–883, 113–117. [Google Scholar] [CrossRef]
- Chu, C.Y.; Zhang, F.B.; Zhu, C.Y.; Fu, T.T.; Ma, Y.G. Mass transfer characteristics of CO2 absorption into 1-butyl-3-methylimidazolium tetrafluoroborate aqueous solution in microchannel. Int. J. Heat Mass Trans. 2019, 128, 1064–1071. [Google Scholar] [CrossRef]
- Li, F.F.; Bai, Y.G.; Zeng, S.J.; Liang, X.D.; Wang, H.; Huo, F.; Zhang, X.P. Protic ionic liquids with low viscosity for efficient and reversible capture of carbon dioxide. Int. J. Greenh. Gas Control 2019, 90, 102801. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, D.; Huang, Y.; Dong, H.F.; Zhang, X.P.; Zhang, S.J. Gas-liquid mass-transfer properties in CO2 absorption system with ionic liquids. AIChE J. 2014, 60, 2929–2939. [Google Scholar] [CrossRef]
- Alcantara, M.L.; Santos, J.P.; Loreno, M.; Ferreira, P.I.S.; Paredes, M.L.L.; Cardozo-Filho, L.; Silva, A.K.; Lião, L.M.; Pires, C.A.M.; Mattedi, S. Low viscosity protic ionic liquid for CO2/CH4 separation: Thermophysical and high-pressure phase equilibria for diethylammonium butanoate. Fluid Phase Equilibr. 2018, 459, 30–43. [Google Scholar] [CrossRef]
- Li, J.; You, C.J.; Chen, L.F.; Ye, Y.M.; Qi, Z.W.; Sundmacher, K. Dynamics of CO2 Absorption and Desorption Processes in Alkanolamine with Cosolvent Polyethylene Glycol. Ind. Eng. Chem. Res. 2012, 51, 12081–12088. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Zhao, Y.-N.; Li, B.; Yu, B. CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion. Energy Environ. Sci. 2011, 4, 3971. [Google Scholar] [CrossRef]
- Liu, S.D.; Li, H.; Chen, Y.F.; Yang, Z.H.; Wang, H.L.; Ji, X.Y.; Lu, X.H. Improved CO2 separation performance of aqueous choline-glycine solution by partially replacing water with polyethylene glycol. Fluid Phase Equilibr. 2019, 495, 12–20. [Google Scholar] [CrossRef]
- Fillion, J.J.; Bennett, J.E.; Brennecke, J.F. The viscosity and density of ionic liquid + tetraglyme mixtures and the effect of tetraglyme on CO2 Solubility. J. Chem. Eng. Data 2017, 62, 608–622. [Google Scholar] [CrossRef]
- Sairi, N.A.; Ghani, N.A.; Aroua, M.K.; Yusoff, R.; Alias, Y. Low pressure solubilities of CO2 in guanidinium trifluoromethanesulfonate-MDEA systems. Fluid Phase Equilibr. 2015, 385, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Sairi, N.A.; Yusoff, R.; Alias, Y.; Aroua, M.K. Solubilities of CO2 in aqueous N-methyldiethanolamine and guanidinium trifluoromethanesulfonate ionic liquid systems at elevated pressures. Fluid Phase Equilibr. 2011, 300, 89–94. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.H.; Yan, J.Y.; Tu, S.-T. CO2 capture using amine solution mixed with ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Xu, F.; Gao, H.S.; Dong, H.F.; Wang, Z.L.; Zhang, X.P.; Ren, B.Z.; Zhang, S.J. Solubility of CO2 in aqueous mixtures of monoethanolamine and dicyanamide-based ionic liquids. Fluid Phase Equilibr. 2014, 365, 80–87. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, F.; Huang, K.; Ma, J.-W.; Wu, Y.-T.; Zhang, Z.-B. Absorption of CO2 in amino acid ionic liquid (AAIL) activated MDEA solutions. Int. J. Greenh. Gas Control 2013, 19, 379–386. [Google Scholar] [CrossRef]
- Baj, S.; Siewniak, A.; Chrobok, A.; Krawczyk, T.; Sobolewski, A. Monoethanolamine and ionic liquid aqueous solutions as effective systems for CO2 capture. J. Chem. Technol. Biotechnol. 2013, 88, 1220–1227. [Google Scholar] [CrossRef]
- Bhawna; Pandey, A.; Pandey, S. Superbase-added choline chloride-based deep eutectic solvents for CO2 capture and sequestration. ChemistrySelect 2017, 2, 11422–11430. [Google Scholar] [CrossRef]
- Huang, K.; Chen, F.-F.; Tao, D.-J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. 2017, 5, 67–73. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, C.-G.; Wu, Y.-T.; Wang, Y.-T.; Li, A.-M.; Zhang, Z.-B. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem. Eng. J. 2010, 160, 691–697. [Google Scholar]
- Babamohammadi, S.; Shamiri, A.; Aroua, M.K. A review of CO2 capture by absorption in ionic liquid-based solvents. Rev. Chem. Eng. 2015, 31, 383–412. [Google Scholar] [CrossRef]
- Lian, S.H.; Song, C.F.; Liu, Q.L.; Duan, E.H.; Ren, H.W.; Kitamura, Y. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Oncsik, T.; Mitschke, B.; MacFarlane, D.R. Base-rich diamino protic ionic liquid mixtures for enhanced CO2 capture. Sep. Purif. Technol. 2018, 196, 27–31. [Google Scholar] [CrossRef]
- Huang, Y.J.; Cui, G.K.; Zhao, Y.L.; Wang, H.Y.; Li, Z.Y.; Dai, S.; Wang, J.J. Reply to the correspondence on “Preorganization and cooperation for highly efficient and reversible capture of low-concentration CO2 by ionic liquids”. Angew. Chem. 2019, 58, 386–389. [Google Scholar] [CrossRef]
- Yasaka, Y.; Kimura, Y. Effect of temperature and water concentration on CO2 absorption by tetrabutylphosphonium formate ionic liquid. J. Chem. Eng. Data 2016, 61, 837–845. [Google Scholar] [CrossRef]
- Simon, N.M.; Zanatta, M.; dos Santos, F.P.; Corvo, M.C.; Cabrita, E.J.; Dupont, J. Carbon dioxide capture by aqueous ionic liquid solutions. ChemSusChem 2017, 10, 4927–4933. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, K.H.; Huangpu, L. Experiments and modeling of absorption of CO2 by amino-cation and amino-anion dual functionalized ionic liquid with the addition of aqueous medium. J. Chem. Eng. Data 2017, 62, 3732–3743. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. Assessment of carbon dioxide solubility in ionic liquid/toluene/water systems by extended PR and PC-SAFT EOSs: Carbon capture implication. J. Mol. Liq. 2019, 275, 323–337. [Google Scholar] [CrossRef]
- Huang, Q.S.; Jing, G.H.; Zhou, X.B.; Lv, B.H.; Zhou, Z.M. A novel biphasic solvent of amino-functionalized ionic liquid for CO2 capture: High efficiency and regenerability. J. CO2 Util. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Qian, Y.H.; Jing, G.H.; Lv, B.H.; Zhou, Z.M. Exploring the general characteristics of amino-acid-functionalized ionic liquids through experimental and quantum chemical calculations. Energy Fuels 2017, 31, 4202–4210. [Google Scholar] [CrossRef]
- Zhou, X.B.; Jing, G.H.; Liu, F.; Lv, B.H.; Zhou, Z.M. Mechanism and kinetics of CO2 absorption into an aqueous solution of a triamino-functionalized ionic liquid. Energy Fuels 2017, 31, 1793–1802. [Google Scholar] [CrossRef]
- Taheri, M.; Dai, C.N.; Lei, Z.G. CO2 capture by methanol, ionic liquid, and their binary mixtures: Experiments, modeling, and process simulation. AIChE J. 2018, 64, 2168–2180. [Google Scholar] [CrossRef]
- Li, X.Y.; Hou, M.Q.; Zhang, Z.F.; Han, B.X.; Yang, G.Y.; Wang, X.L.; Zou, L.Z. Absorption of CO2 by ionic liquid/polyethylene glycol mixture and the thermodynamic parameters. Green Chem. 2008, 10, 879. [Google Scholar] [CrossRef]
- Li, J.; Dai, Z.D.; Usman, M.; Qi, Z.W.; Deng, L.Y. CO2 /H2 separation by amino-acid ionic liquids with polyethylene glycol as co-solvent. Int. J. Greenh. Gas Con. 2016, 45, 207–215. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, H. Carbon dioxide capture by diethylenetriamine hydrobromide in nonaqueous systems and phase-change formation. Energy Fuels 2017, 31, 5363–5375. [Google Scholar] [CrossRef]
- Lepre, L.F.; Pison, L.; Siqueira, L.J.A.; Ando, R.A.; Costa Gomes, M.F. Improvement of carbon dioxide absorption by mixing poly(ethylene glycol) dimethyl ether with ammonium-based ionic liquids. Sep. Purif. Technol. 2018, 196, 10–19. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Taheri, M.; Yu, G.Q.; Zhu, J.Q.; Lei, Z.G. Experiments, modeling, and simulation of CO2 dehydration by ionic liquid, triethylene glycol, and their binary mixtures. Ind. Eng. Chem. Res. 2019, 58, 15588–15597. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.L.; Li, S.J.; Li, W. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Y.; Jin, X.B.; Zhao, D.; Chen, G.Z. Chloride ion enhanced thermal stability of carbon dioxide captured by monoethanolamine in hydroxyl imidazolium based ionic liquids. Energy Environ. Sci. 2011, 4, 2125. [Google Scholar] [CrossRef]
- Fu, D.; Hao, H.M.; Liu, F. Experiment and model for the viscosity of carbonated 2-amino-2-methyl-1-propanol-monoethanolamine and 2-amino-2-methyl-1-propanol-diethanolamine aqueous solution. J. Mol. Liq. 2013, 188, 37–41. [Google Scholar] [CrossRef]
- Goodrich, B.F.; de la Fuente, J.C.; Gurkan, B.E.; Zadigian, D.J.; Price, E.A.; Huang, Y.; Brennecke, J.F. Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide. Ind. Eng. Chem. Res. 2011, 50, 111–118. [Google Scholar] [CrossRef]
- Liu, F.; Jing, G.H.; Zhou, X.B.; Lv, B.H.; Zhou, Z.M. Performance and mechanisms of triethylene tetramine (TETA) and 2-Amino-2-methyl-1-propanol (AMP) in aqueous and nonaqueous solutions for CO2 capture. ACS Sustain. Chem. Eng. 2017, 6, 1352–1361. [Google Scholar] [CrossRef]
- Zheng, W.-T.; Huang, K.; Wu, Y.-T.; Hu, X.-B. Protic ionic liquid as excellent shuttle of MDEA for fast capture of CO2. AIChE J. 2018, 64, 209–219. [Google Scholar] [CrossRef]
- Meng, Y.N.; Wang, X.D.; Zhang, F.; Zhang, Z.B.; Wu, Y.T. IL-DMEE Nonwater system for CO2 capture: Absorption performance and mechanism investigations. Energy Fuels 2018, 32, 8587–8593. [Google Scholar] [CrossRef]
- Salleh, R.M.; Jamaludin, S.N. Thermodynamic equilibrium solubility of diethanolamine–N-butyl-1-methylpyrrolidinium dicyanamide [DEABMPYRR DCA] mixtures for carbon dioxide capture. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012010.53. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Al-Ajmi, A.; Vakili-Nezhaad, G.R. Investigation of physico-chemical properties for the 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4])–diethylenetriamine (DETA) system for CO2 capture. J. Solut. Chem. 2019, 48, 578–610. [Google Scholar] [CrossRef]
- Harifi-Mood, A.R.; Mohammadpour, F.; Boczkaj, G. Solvent dependency of carbon dioxide Henry’s constant in aqueous solutions of choline chloride-ethylene glycol based deep eutectic solvent. J. Mol. Liq. 2020, 319, 114173. [Google Scholar] [CrossRef]
- Lin, C.-M.; Leron, R.B.; Caparanga, A.R.; Li, M.-H. Henry’s constant of carbon dioxide-aqueous deep eutectic solvent (choline chloride/ethylene glycol, choline chloride/glycerol, choline chloride/malonic acid) systems. J. Chem. Thermodyn. 2014, 68, 216–220. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.H.; Zhang, H.M.; Ma, J.W.; Jiang, B.; Zhang, L.H. Highly efficient and reversible CO2 capture by task-specific deep eutectic solvents. Ind. Eng. Chem. Res. 2019, 58, 13321–13329. [Google Scholar] [CrossRef]
- Ren, H.W.; Lian, S.H.; Wang, X.; Zhang, Y.; Duan, E.H. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Siani, G.; Tiecco, M.; Di Profio, P.; Guernelli, S.; Fontana, A.; Ciulla, M.; Canale, V. Physical absorption of CO2 in betaine/carboxylic acid-based natural deep eutectic solvents. J. Mol. Liq. 2020, 315, 113708. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Garea, A.; Irabien, Á. Hybrid solvent ([emim][Ac]+water) to improve the CO2 capture efficiency in a PVDF hollow fiber contactor. ACS Sustain. Chem. Eng. 2017, 5, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.L.; Deng, Z.Y.; Ma, J.Y.; Qin, Y.H.; Zhang, Y.; Luo, Y.B.; Wu, Z.K. Comparison of mass transfer coefficients and desorption rates of CO2 absorption into aqueous MEA + ionic liquids solution. Chem. Eng. Res. Des. 2017, 117, 66–72. [Google Scholar] [CrossRef]
- Ma, C.Y.; Sarmad, S.; Mikkola, J.-P.; Ji, X.Y. Development of low-cost deep eutectic solvents for CO2 capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]





| DES-Based Hybrid Solvents | Amount/Ratio | Temperature (K) | Pressure (MPa) | CO2 Solubility (mol CO2/kg DES) | CO2 Solubility (mol CO2/mol DES) | Viscosity (mPa·s) | Ref. |
|---|---|---|---|---|---|---|---|
| DES–H2O | |||||||
| [BHDE][Cl]-GLY-H2O | 1:3:0.11 | 298.15 | 0.23–2.02 | 0.037–0.21 | 32.76–17.11 | [9] | |
| [BTMA][Cl]-GLY-H2O | 1:2:0.05 | 298.15 | 0.21–2.02 | 0.044–0.29 | 70.76–26.81 | [9] | |
| [BTMA][Cl]-GLY-H2O | 1:2:0.11 | 298.15 | 0.26–2.03 | 0.016–0.33 | 22.19–14.41 | [9] | |
| [TEMA][Cl]-GLY-H2O | 1:2:0.05 | 298.15 | 0.23–1.98 | 0.009–0.66 | 90.98–23.56 | [9] | |
| [TEMA][Cl]-GLY-H2O | 1:2:0.11 | 298.15 | 0.14–1.74 | 0.025–0.66 | 48.63–19.31 | [9] | |
| [L-Arg]-GLY 1:6-H2O | 10, 20, 30, 40, 50, 60 wt % | 303.15–353.15 | 0.1 | 434.2–26.3, 179.3–19.5, 45.2–7.2, 17.7–4.4, 8.0–3.6, 4.6–3.0 | [57] | ||
| DES–organic | |||||||
| [Ch][Cl]-GLY-AC | 1:1:1 | 298.15 | 0.26–2.01 | 0.052–0.43 | 138.51–28.81 | [9] | |
| [MTPP][Br]-LEV-AC | 1:3:0.03 | 298.15 | 0.29–2.06 | 0.18–1.32 | 40.12–16.16 | [9] | |
| [Ch][Cl]-Urea 1:2 + TBD | 1:10 | 333.15 | 0.1 | 0.42 | 0.68 | [25] | |
| [Ch][Cl]-Urea 1:2 + DBU | 1:10 | 333.15 | 0.1 | 0.56 | 1.21 | [25] | |
| [Ch][Cl]-Urea 1:2 + DBN | 1:10 | 333.15 | 0.1 | 0.76 | 1.11 | [25] | |
| [Ch][Cl]-Urea 1:2-GLY + TBD | 5:40:10 | 333.15 | 0.1 | 0.4 | 0.66 | [25] | |
| [Ch][Cl]-Urea 1:2-GLY + DBU | 5:40:10 | 333.15 | 0.1 | 0.45 | 0.81 | [25] | |
| [Ch][Cl]-Urea 1:2-GLY + DBN | 5:40:10 | 333.15 | 0.1 | 0.27 | 0.40 | [25] | |
| [Ch][Cl]-EG 1:2 + DBU | 1:10 | 333.15 | 0.1 | 0.7 | 1.16 | [25] | |
| [Ch][Cl]-EG 1:2 + DBN | 1:10 | 333.15 | 0.1 | 0.77 | 1.06 | [25] | |
| [Ch][Cl]-EG 1:2 + TBD | 1:10 | 333.15 | 0.1 | 0.63 | 0.95 | [25] | |
| [Ch][Cl]-EG 1:2-GLY + DBU | 5:40:10 | 333.15 | 0.1 | 0.68 | 1.16 | [25] | |
| [Ch][Cl]-EG 1:2 + DBU | 1:10 | 298.15 | 0.1 | 0.83 | 1.41 | [25] | |
| [Ch][Cl]-EG 1:2 + DBN | 1:10 | 298.15 | 0.1 | 0.86 | 1.19 | [25] | |
| [Ch][Cl]-EG 1:2 + TBD | 1:10 | 298.15 | 0.1 | 0.76 | 1.17 | [25] | |
| [Ch][Cl]-EG 1:2-GLY + DBU | 5:40:10 | 298.15 | 0.1 | 0.64 | 1.12 | [25] | |
| [Ch][Cl]-EG 1:2-GLY + DBN | 5:40:10 | 298.15 | 0.1 | 0.74 | 1.05 | [25] | |
| [Ch][Cl]-EG 1:2-GLY + TBD | 5:40:10 | 298.15 | 0.1 | 0.73 | 1.17 | [25] | |
| [Ch][Cl]-MEA 1:2 + DBU | 1:10 | 298.15 | 0.1 | 3.54 | 2.86 | [25] | |
| [Ch][Cl]-MEA 1:2 + DBN | 1:10 | 298.15 | 0.1 | 5.11 | 6.70 | [25] | |
| [Ch][Cl]-MEA 1:4 + TBD | 1:10 | 298.15 | 0.1 | 3.91 | 5.77 | [25] | |
| [Ch][Cl]-MEA 1:2-GLY + DBU | 5:40:10 | 298.15 | 0.1 | 3.26 | 5.46 | [25] | |
| [Ch][Cl]-MEA 1:2-GLY + DBN | 5:40:10 | 298.15 | 0.1 | 1.67 | 2.28 | [25] | |
| [Ch][Cl]-MEA 1:2-GLY + TBD | 5:40:10 | 298.15 | 0.1 | 3.63 | 5.56 | [25] | |
| [BMIM][Cl]-Im + DBN | 1:1:1 | 298.15–328.15 | 0.1 | 0.81–1.02 | [56] | ||
| [BMIM][Cl]-Im + DBN | 1:1:2 | 298.15–328.15 | 0.1 | 0.88–0.97 | [56] | ||
| [BMIM][Cl]-Im + DBN | 1:2:1 | 298.15–328.15 | 0.1 | 0.91–1.07 | [56] | ||
| DES-H2O | T (K) | Henry’s Constant | Ref. |
|---|---|---|---|
| [Ch][Cl]-EG + H2O (10, 20, 30, 40, 50, 60, 70, 80, 90 mol%) | 303.15–313.15 | 27–296 | [54] |
| [Ch][Cl]-EG + H2O (20, 40, 60, 80 wt %) | 303.15–313.15 | 3965.5–2805.5 | [55] |
| [Ch][Cl]-GLY + H2O (20, 40, 60, 80 wt %) | 303.15–313.15 | 3818.8–3185.2 | [55] |
| [Ch][Cl]-MA + H2O (20, 40, 60, 80 wt %) | 303.15–313.15 | 4021.6–2946.2 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dai, Z.; Dai, F.; Ji, X. Ionic Liquids/Deep Eutectic Solvents-Based Hybrid Solvents for CO2 Capture. Crystals 2020, 10, 978. https://doi.org/10.3390/cryst10110978
Liu Y, Dai Z, Dai F, Ji X. Ionic Liquids/Deep Eutectic Solvents-Based Hybrid Solvents for CO2 Capture. Crystals. 2020; 10(11):978. https://doi.org/10.3390/cryst10110978
Chicago/Turabian StyleLiu, Yanrong, Zhengxing Dai, Fei Dai, and Xiaoyan Ji. 2020. "Ionic Liquids/Deep Eutectic Solvents-Based Hybrid Solvents for CO2 Capture" Crystals 10, no. 11: 978. https://doi.org/10.3390/cryst10110978