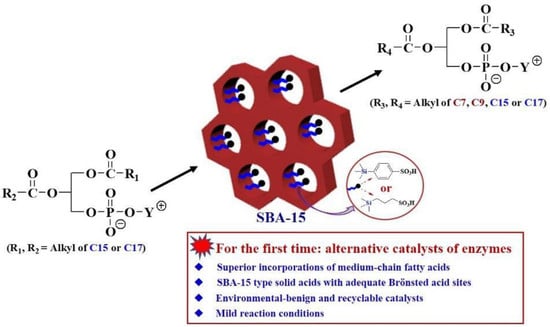
Efficient Production of Medium-Chain Structured Phospholipids over Mesoporous Organosulfonic Acid-Functionalized SBA-15 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Production of SPLs with MCFAs
2.3. Recyclability of the Sulfonic Acid-Modified SBA-15 Catalysts
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
3.5. Analysis of the Fatty Acid Composition
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, W.; Chen, J. Heterogeneous interesterification of triacylglycerols catalyzed by using potassium-doped alumina as a solid catalyst. J. Agric. Food Chem. 2014, 62, 10414–10421. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Jayasooriya, A.P.; Madhujith, T. Optimization of the production of structured lipid by enzymatic interesterification from coconut (Cocos nucifera) oil and sesame (Sesamum indicum) oil using Response Surface Methodology. LWT-Food Sci. Technol. 2019, 101, 723–730. [Google Scholar] [CrossRef]
- Xie, W.; Hu, P. Production of structured lipids containing medium-chain fatty acids by soybean oil acidolysis using SBA-15-pr-NH2-HPW catalyst in a heterogeneous manner. Org. Process Res. Dev. 2016, 20, 637–645. [Google Scholar] [CrossRef]
- Wang, X.; Zou, S.; Miu, Z.; Jin, Q.; Wang, X. Enzymatic preparation of structured triacylglycerols with arachidonic and palmitic acids at the sn-2 position for infant formula use. Food Chem. 2019, 283, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, S.H. Effects of organic solvents on transesterification of phospholipids using phospholipase A2 and lipase. Food Sci. Biotechnol. 2014, 23, 1207–1211. [Google Scholar] [CrossRef]
- Reddy, J.R.C.; Vijeeta, T.; Karuna, M.S.L.; Rao, B.V.S.K.; Prasad, R.B.N. Lipase-catalyzed preparation of palmitic and stearic acid-rich phosphatidylcholine. J. Am. Oil Chem. Soc. 2005, 82, 727–730. [Google Scholar] [CrossRef]
- Lee, H.S.; Sung, D.K.; Kim, S.H.; Choi, W.I.; Hwang, E.T.; Choi, D.J.; Chang, J.H. Controlled release of astaxanthin from nanoporous silicified-phospholipids assembled boron nitride complex for cosmetic applications. Appl. Surf. Sci. 2017, 424, 15–19. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Recent research trends on the enzymatic synthesis of structured lipids. J. Food Sci. 2015, 80, C1713–C1724. [Google Scholar] [CrossRef]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar]
- Chojnacka, A.; Gładkowski, W.; Gliszczyńska, A.; Niezgoda, N.; Kiełbowicz, G.; Wawrzeńczyk, C. Synthesis of structured phosphatidylcholine containing punicic acid by the lipase-catalyzed transesterification with pomegranate seed oil. Catal. Commun. 2016, 75, 60–64. [Google Scholar] [CrossRef]
- Zhao, T.T.; No, D.S.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; García, H.S.; Vernon-Carter, E.J. Phosphatidylcholine enrichment with medium chain fatty acids by immobilized phospholipase A1-catalyzed acidolysis. Biotechnol. Progr. 2013, 29, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Akinfalabi, S.I.; Rashid, U.; Choong Shean, T.Y.; Nehdi, I.A.; Sbihi, H.M.; Gewik, M.M. Esterification of palm fatty acid distillate for biodiesel production catalyzed by synthesized kenaf seed cake-based sulfonated catalyst. Catalysts 2019, 9, 482. [Google Scholar] [CrossRef]
- Gaidukevic, J.; Barkauskas, J.; Malaika, A.; Rechnia-Goracy, P.; Mozdzynska, A.; Jasulaitiene, V.; Kozlowski, M. Modified graphene-based materials as effective catalysts for transesterification of rapeseed oil to biodiesel fuel. Chin. J. Catal. 2018, 39, 1633–1645. [Google Scholar] [CrossRef]
- Fu, X.; Li, D.; Chen, J.; Zhang, Y.; Huang, W.; Zhu, Y.; Yang, J.; Zhang, C. A microalgae residue based carbon solid acid catalyst for biodiesel production. Bioresource Technol. 2013, 146, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous catalysts for biodiesel production. Energy Fuel 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Garcia, C.M.; Teixeira, S.; Marciniuk, L.L.; Schuchardt, U. Transesterification of soybean oil catalyzed by sulfated zirconia. Bioresource Technol. 2008, 99, 6608–6613. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ito, S.; Hara, K.; Fukuoka, A. Conversion of glycerol to acrolein by mesoporous sulfated zirconia-silica catalyst. Chin. J. Catal. 2017, 38, 420–425. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Fan, M.; Sun, W.; Jiang, P.; Dong, Y. Direct and postsynthesis of tin-incorporated SBA-15 functionalized with sulfonic acid for efficient biodiesel production. Fuel 2019, 235, 426–432. [Google Scholar] [CrossRef]
- Zuo, D.; Lane, J.; Culy, D.; Schultz, M.; Pullar, A.; Waxman, M. Sulfonic acid functionalized mesoporous SBA-15 catalysts for biodiesel production. Appl. Catal. B-Environ. 2013, 129, 342–350. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A-Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Cattaneo, A.S.; Ferrara, C.; Villa, D.C.; Angioni, S.; Milanese, C.; Capsoni, D.; Grandi, S.; Mustarelli, P.; Allodi, V.; Mariotto, G.; et al. SBA-15 mesoporous silica highly functionalized with propylsulfonic pendants: A thorough physico-chemical characterization. Microporous Mesoporous Mater. 2016, 219, 219–229. [Google Scholar] [CrossRef]
- Jin, H.; Ansari, M.B.; Park, S. Sulfonic acid functionalized mesoporous ZSM-5: Synthesis, characterization and catalytic activity in acidic catalysis. Catal. Today 2015, 245, 116–121. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chu, Q.; Chen, J.; Hou, W.; Yu, H.; Wang, P.; Liu, R.; Song, G.; Zhu, H.; Zhao, P. Enhancement of catalytic activity by homo-dispersing S2O82−-Fe2O3 nanoparticles on SBA-15 through ultrasonic adsorption. Chin. J. Catal. 2018, 39, 955–963. [Google Scholar] [CrossRef]
- Srivastava, R. An efficient, eco-friendly process for aldol and Michael reactions of trimethylsilyl enolate over organic base-functionalized SBA-15 catalysts. J. Mol. Catal. A-Chem. 2007, 264, 146–152. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Robinson, N.; Barbero, B.; Durndell, L.J.; Manayil, J.C.; Parlett, C.M.A.; D’Agostino, C.; Wilson, K.; Lee, A.F. Unravelling mass transport in hierarchically porous catalysts. J. Mater. Chem. A 2019, 7, 11814–11825. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Yu, H.; Hua, D.; Zhou, S. Enhanced catalytic performance of molybdenum-doped mesoporous SBA-15 for metathesis of 1-butene and ethene to propene. Catal. Sci. Technol. 2014, 4, 4010–4019. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Zhang, Z.; Cui, L.; Jia, J.; Zhou, D.; Zhu, B. An excellent solid acid catalyst derived from microalgae residue for fructose dehydration into 5-hydroxymethylfurural. ChemistrySelect 2019, 4, 1259–1265. [Google Scholar] [CrossRef]
- Amoozadeh, A.; Rahmani, S. Nano-WO3-supported sulfonic acid: New, efficient and high reusable heterogeneous nano catalyst. J. Mol. Catal. A-Chem. 2015, 396, 96–107. [Google Scholar] [CrossRef]
- Geng, L.; Wang, Y.; Yu, G.; Zhu, Y. Efficient carbon-based solid acid catalysts for the esterification of oleic acid. Catal. Commun. 2011, 13, 26–30. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, H.; Ma, Y.; Zhang, R. High selective production of 5-hydroxymethylfurfural from fructose by sulfonic acid functionalized SBA-15 catalyst. Compos. Part B-Eng. 2019, 156, 88–94. [Google Scholar] [CrossRef]
- Veisi, H.; Sedrpoushan, A.; Faraji, A.R.; Heydari, M.; Hemmati, S.; Fatahi, B. A mesoporous SBA-15 silica catalyst functionalized with phenylsulfonic acid groups (SBA-15-Ph-SO3H) as a novel hydrophobic nanoreactor solid acid catalyst for a one-pot three-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones and triazolo[1,2-a]indazole-triones. RSC Adv. 2015, 5, 68523–68530. [Google Scholar] [CrossRef]
- Thapa, I.; Mullen, B.; Saleem, A.; Leibig, C.; Baker, R.T.; Giorgi, J.B. Efficient green catalysis for the conversion of fructose to levulinic acid. Appl. Catal. A-Gen. 2017, 539, 70–79. [Google Scholar] [CrossRef]
- Rao, G.S.; Rajan, N.P.; Sekhar, M.H.; Ammaji, S.; Chary, K.V.R. Porous zirconium phosphate supported tungsten oxide solid acid catalysts for the vapour phase dehydration of glycerol. J. Mol. Catal. A-Chem. 2014, 395, 486–493. [Google Scholar] [CrossRef]
- Chen, F.R.; Coudurier, G.; Joly, J.F.; Vedrine, J.C. Superacid and catalytic properties of sulfated zirconia. J. Catal. 1993, 143, 616–626. [Google Scholar] [CrossRef]
- Morterra, C.; Cerrato, G.; Emanuel, C.; Bolis, V. On the surface acidity of some sulfate-doped ZrO2 catalysts. J. Catal. 1993, 142, 349–367. [Google Scholar] [CrossRef]
- Miao, S.; Shanks, B.H. Esterification of biomass pyrolysis model acids over sulfonic acid-functionalized mesoporous silicas. Appl. Catal. A-Gen. 2009, 359, 113–120. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, C. Production of medium-chain structured lipids using dual acidic ionic liquids supported on Fe3O4@SiO2 composites as magnetically recyclable catalysts. LWT Food Sci. Technol. 2018, 93, 71–78. [Google Scholar] [CrossRef]
- Li, Z.; Ward, O.P. Lipase-catalyzed esterification of glycerol and n-3 polyunsaturated fatty acid concentrate in organic solvent. J. Am. Oil Chem. Soc. 1993, 70, 745–748. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.; Liu, W.; Cheng, B.; Cao, X.; Mao, J.; Zhu, S. Hydrothermal preparation and characterization of novel corncob-derived solid acid catalysts. J. Agric. Food Chem. 2014, 62, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Tan, M.; Jiang, B.; Zheng, J.; Tsubaki, N.; Wu, M. Monodispersed hollow SO3H-functionalized carbon/silica as efficient solid acid catalyst for esterification of oleic acid. ACS Appl. Mater. Int. 2015, 7, 26767–26775. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.S.; Forrester, A.M.S.; de la Cruz, M.H.C.; da Silva, C.T.; Lachter, E.R. Comparative performance of niobium phosphates in liquid phase anisole benzylation with benzyl alcohol. Catal. Commun. 2008, 9, 1959–1965. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Marsaoui, N.; Laplante, S.; Raies, A.; Naghmouchi, K. Incorporation of omega-3 polyunsaturated fatty acids into soybean lecithin: Effect of amines and divalent cations on transesterification by lipases. World J. Microbiol. Biotechnol. 2013, 29, 2233–2238. [Google Scholar] [CrossRef]
- Kaki, S.S.; Balakrishna, M.; Prasad, R.B.N. Enzymatic synthesis and characterization of 1-lipoyl-2-palmitoyl phosphatidylcholine: A novel phospholipid containing lipoic acid. Eur. J. Lipid Sci. Technol. 2014, 116, 1347–1353. [Google Scholar] [CrossRef]
- Hartman, L.; Lago, R.C. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pr. 1973, 22, 475–476. [Google Scholar]
- De Martini Soares, F.A.S.; da Silva, R.C.; da Silva, K.C.G.; Lourenço, M.B.; Soares, D.F.; Gioielli, L.A. Effects of chemical interesterification on physicochemical properties of blends of palm stearin and palm olein. Food Res. Int. 2009, 42, 1287–1294. [Google Scholar] [CrossRef]









| FA | C8:0 | C10:0 | C16:0 c | C18:0 c | C18:1 c | C18:2 c |
|---|---|---|---|---|---|---|
| SPL (Ph b) | 26.17 ± 0.44 | 0 | 6.16 ± 0.12 | 3.52 ± 1.03 | 49.43 ± 0.54 | 14.72 ± 0.23 |
| 0 | 30.92 ± 0.83 | 4.99 ± 0.33 | 2.71 ± 0.74 | 44.47 ± 0.82 | 16.91 ± 0.35 | |
| SPL (Pr b) | 23.22 ± 0.25 | 0 | 6.74 ± 0.27 | 3.94 ± 0.55 | 45.36 ± 0.90 | 20.74 ± 0.41 |
| 0 | 27.82 ± 0.87 | 4.58 ± 0.29 | 3.81 ± 1.01 | 45.57 ± 0.37 | 18.22 ± 0.53 | |
| SL | 0 | 0 | 7.39 ± 0.31 | 4.18 ± 0.53 | 66.36 ± 0.46 | 22.05 ± 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, S.; Cai, W.; Yin, F.; Jia, J.; Zhou, D.; Zhu, B. Efficient Production of Medium-Chain Structured Phospholipids over Mesoporous Organosulfonic Acid-Functionalized SBA-15 Catalysts. Catalysts 2019, 9, 770. https://doi.org/10.3390/catal9090770
Zhang J, Yang S, Cai W, Yin F, Jia J, Zhou D, Zhu B. Efficient Production of Medium-Chain Structured Phospholipids over Mesoporous Organosulfonic Acid-Functionalized SBA-15 Catalysts. Catalysts. 2019; 9(9):770. https://doi.org/10.3390/catal9090770
Chicago/Turabian StyleZhang, Jianghua, Shasha Yang, Weijie Cai, Fawen Yin, Jin Jia, Dayong Zhou, and Beiwei Zhu. 2019. "Efficient Production of Medium-Chain Structured Phospholipids over Mesoporous Organosulfonic Acid-Functionalized SBA-15 Catalysts" Catalysts 9, no. 9: 770. https://doi.org/10.3390/catal9090770