Defect-Rich Nickel Nanoparticles Supported on SiC Derived from Silica Fume with Enhanced Catalytic Performance for CO Methanation
Abstract
:1. Introduction
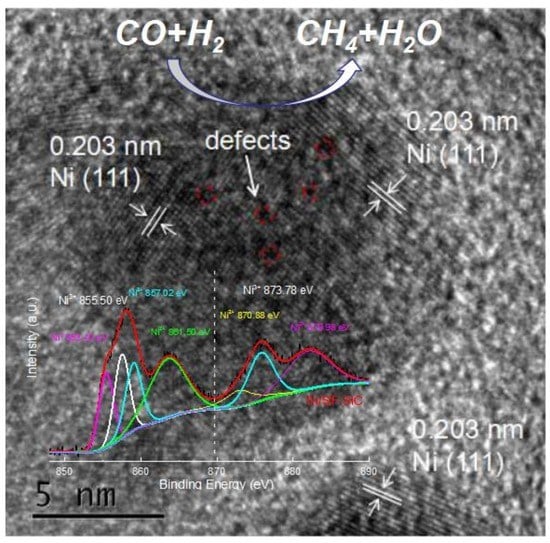
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Physical Characterization of the Synthesized Samples
3.3. CO Methanation Performance Test of Powdered Catalyst
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Wang, C.-A.; Tie, S. Synthesis of bamboo-like SiC whiskers from waste silica fume. Cryst. Res. Technol. 2014, 49, 290–297. [Google Scholar] [CrossRef]
- Moen, M.; Halvorsen, T.; Mørk, K.; Velken, S. Recycling of silicon metal powder from industrial powder waste streams. Met. Powder Rep. 2017, 72, 182–187. [Google Scholar] [CrossRef]
- Zhong, Y.; Shaw, L.L.; Manjarres, M.; Zawrah, M.F. Synthesis of Silicon Carbide Nanopowder Using Silica Fume. J. Am. Ceram. Soc. 2010, 93, 3159–3167. [Google Scholar] [CrossRef]
- Danghyan, V.; Novoa, S.C.; Mukasyan, A.; Wolf, E.E. Pressure dilution, a new method to prepare a stable Ni/fumed silica catalyst for the dry reforming of methane. Appl. Catal. B Environ. 2018, 234, 178–186. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Chen, K.; Li, W.; Gao, Y.; Fang, M.; Liu, Y.G.; Wu, X. Synthesis of Si3N4 powder with tunable α/β-Si3N4 content from waste silica fume using carbothermal reduction nitridation. Powder Technol. 2014, 252, 51–55. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Lei, X.; Ren, Y.; Wu, J. Preparation of high silica microporous zeolite SSZ-13 using solid waste silica fume as silica source. Adv. Powder Technol. 2018, 29, 1112–1118. [Google Scholar] [CrossRef]
- Kumar, P.; Mal, N.; Oumi, Y.; Yamana, K.; Sano, T. Mesoporous materials prepared using coal fly ash as the silicon and aluminium source. J. Mater. Chem. 2001, 11, 3285–3290. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, L.; Liang, J.; Han, Y.; Lu, Y.; Zhu, Y.; Qian, Y. Porous silicon nano-aggregate from silica fume as an anode for high-energy lithium-ion batteries. RSC Adv. 2016, 6, 30577–30581. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.; Tian, Z.; Han, Y.; Zhang, Y.; Zhou, T.; Kang, L.; Dan, J.; Guo, X.; Yu, F.; et al. Two-Dimensional Layered Double Hydroxide Derived from Vermiculite Waste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation. Catalysts 2017, 7, 79. [Google Scholar] [CrossRef]
- Li, P.; Yu, F.; Altaf, N.; Zhu, M.; Li, J.; Dai, B.; Wang, Q. Two-Dimensional Layered Double Hydroxides for Reactions of Methanation and Methane Reforming in C1 Chemistry. Materials 2018, 11, 221. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, Y.; Tian, Y.; Gu, F.; Zhong, Z.; Su, F. Ordered Mesoporous Ni–Fe–Al Catalysts for CO Methanation with Enhanced Activity and Resistance to Deactivation. Ind. Eng. Chem. Res. 2017, 56, 9809–9820. [Google Scholar] [CrossRef]
- Bian, Z.; Xin, Z.; Meng, X.; Tao, M.; Lv, Y.; Gu, J. Effect of Citric Acid on the Synthesis of CO Methanation Catalysts with High Activity and Excellent Stability. Ind. Eng. Chem. Res. 2017, 56, 2383–2392. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Zhu, M.; Tian, Z.; Dan, J.; Li, J.; Dai, B.; Yu, F. Ultralow-weight loading Ni catalyst supported on two-dimensional vermiculite for carbon monoxide methanation. Chin. J. Chem. Eng. 2018, 26, 1873–1878. [Google Scholar] [CrossRef]
- Tan, C.; Liu, J.; Zhang, H.; Wang, J.; Li, S.; Song, J.; Zhang, Y.; Zhang, S. Low temperature synthesis of 2H-SiC powders via molten-salt-mediated magnesiothermic reduction. Ceram. Int. 2017, 43, 2431–2437. [Google Scholar] [CrossRef]
- Gao, P.-C.; Lei, Y.; Pérez, A.F.; Rajoua, K.; Zitoun, D.; Favier, F. New topotactic synthetic route to mesoporous silicon carbide. J. Mater. Chem. 2011, 21, 15798–15805. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.; Peng, S.; Zhou, X.; Zhang, H.; Zhou, X.; Li, B. KD-S SiCf/SiC composites with BN interface fabricated by polymer infiltration and pyrolysis process. J. Adv. Ceram. 2018, 7, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Cai, L. Fabrication and oxidation resistance of mullite/yttrium silicate multilayer coatings on C/SiC composites. J. Adv. Ceram. 2017, 6, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Magnani, G.; Galvagno, S.; Sico, G.; Portofino, S.; Freda, C.; Burresi, E. Sintering and mechanical properties of β-SiC powder obtained from waste tires. J. Adv. Ceram. 2016, 5, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Yang, Y.; Huang, Z.; Liu, G.; Zhang, H.; Jiang, F.; Wei, Y.; Jiao, Z. Investigation on the laser ablation of SiC ceramics using micro-Raman mapping technique. J. Adv. Ceram. 2016, 5, 253–261. [Google Scholar] [CrossRef]
- Zhang, G.; Peng, J.; Sun, T.; Wang, S. Effects of the oxidation extent of the SiC surface on the performance of Ni/SiC methanation catalysts. Chin. J. Catal. 2013, 34, 1745–1755. [Google Scholar] [CrossRef]
- Jin, G.; Gu, F.; Liu, Q.; Wang, X.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. Highly stable Ni/SiC catalyst modified by Al2O3 for CO methanation reaction. RSC Adv. 2016, 6, 9631–9639. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.-Q.; Wang, Y.-Y.; Guo, X.-Y. Synthetic natural gas from CO hydrogenation over silicon carbide supported nickel catalysts. Fuel Process. Technol. 2011, 92, 2293–2298. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.; Peng, W.; Zhang, Q.; Luo, G.; Wei, F. Novel hierarchical Ni/MgO catalyst for highly efficient CO methanation in a fluidized bed reactor. AIChE J. 2017, 63, 2141–2152. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Chen, D.; Christensen, K.; Ochoafernandez, E.; Yu, Z.; Totdal, B.; Latorre, N.; Monzon, A.; Holmen, A. Synthesis of carbon nanofibers: Effects of Ni crystal size during methane decomposition. J. Catal. 2005, 229, 82–96. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, T.; Peng, J.; Wang, S.; Wang, S. A comparison of Ni/SiC and Ni/Al2O3 catalyzed total methanation for production of synthetic natural gas. Appl. Catal. A Gen. 2013, 462–463, 75–81. [Google Scholar] [CrossRef]
- Liu, Q.H.; Dong, X.F.; Lin, W.M. Highly selective CO methanation over amorphous Ni–Ru–B/ZrO2 catalyst. Chin. Chem. Lett. 2009, 20, 889–892. [Google Scholar] [CrossRef]
- Liu, S.-S.; Jin, Y.-Y.; Han, Y.; Zhao, J.; Ren, J. Highly stable and coking resistant Ce promoted Ni/SiC catalyst towards high temperature CO methanation. Fuel Process. Technol. 2018, 177, 266–274. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Z.; Chen, Y.; Ma, X. Syngas methanation over Ni/SiO2 catalyst prepared by ammonia-assisted impregnation. Int. J. Hydrogen Energy 2017, 42, 27073–27083. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.; Bian, Z.; Xin, Z. Effect of impregnation solvent on Ni dispersion and catalytic properties of Ni/SBA-15 for CO methanation reaction. Fuel 2016, 165, 289–297. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.; Dan, J.; Kang, L.; Lai, L.; Cai, X.; Zhang, J.; Yu, F.; Tian, Z.; Dai, B. Two-dimensional porous SiO2 nanomesh supported high dispersed Ni nanoparticles for CO methanation. Chem. Eng. J. 2017, 326, 774–780. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Ni/Al2O3 catalysts for CO methanation: Effect of Al2O3 supports calcined at different temperatures. J. Energy Chem. 2013, 22, 919–927. [Google Scholar] [CrossRef]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F.; et al. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Wang, Y.; Guo, X. Synthesis of natural gas from CO methanation over SiC supported Ni–Co bimetallic catalysts. Catal. Commun. 2013, 31, 5–10. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, C.; Bao, J.; Li, S.; Qi, D.; Wang, Q.; Zhao, B.; Hu, T.; Fan, L.; Fan, B. Ni-based catalyst with enhanced Ni-support interaction for highly efficient CO methanation. Catal. Sci. Technol. 2018, 8, 3474–3483. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, J.; Sha, G.; Li, C.; Zhang, B.; Su, D.; Williams, C.T.; Liang, C. Silicon–nickel intermetallic compounds supported on silica as a highly efficient catalyst for CO methanation. Catal. Sci. Technol. 2014, 4, 53–61. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Kim, M.S.; Park, E.D. A highly loaded Ni@SiO2 core–shell catalyst for CO methanation. Appl. Catal. A Gen. 2016, 513, 98–105. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Tian, Z.; Zhu, M.; Wang, F.; Li, J.; Dai, B.; Yu, F.; Qiu, H.; Gao, H. Clarification of Active Sites at Interfaces between Silica Support and Nickel Active Components for Carbon Monoxide Methanation. Catalysts 2018, 8, 293. [Google Scholar] [CrossRef]
- Cai, W.-J.; Qian, L.-P.; Yue, B.; He, H.-Y. Rh doping effect on coking resistance of Ni/SBA-15 catalysts in dry reforming of methane. Chin. Chem. Lett. 2014, 25, 1411–1415. [Google Scholar] [CrossRef]
- García-Vargas, J.M.; Valverde, J.L.; de Lucas-Consuegra, A.; Gómez-Monedero, B.; Sánchez, P.; Dorado, F. Precursor influence and catalytic behaviour of Ni/CeO2 and Ni/SiC catalysts for the tri-reforming process. Appl. Catal. A Gen. 2012, 431–432, 49–56. [Google Scholar]
- Zhao, Y.; Jia, X.; Chen, G.; Shang, L.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Ultrafine NiO Nanosheets Stabilized by TiO2 from Monolayer NiTi-LDH Precursors: An Active Water Oxidation Electrocatalyst. J. Am. Chem. Soc. 2016, 138, 6517–6524. [Google Scholar] [CrossRef]
- Sasi, B.; Gopchandran, K.G. Nanostructured mesoporous nickel oxide thin films. Nanotechnology 2007, 18, 115613. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, F.; Li, J.; Chen, K.; Yao, Y.; Li, P.; Zhu, M.; Shi, Y.; Wang, Q.; Guo, X. High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation. Catalysts 2018, 8, 363. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, C.; Zhang, Z.; Liu, D.; Chen, R.; Wang, S. In Situ Exfoliated, N-Doped, and Edge-Rich Ultrathin Layered Double Hydroxides Nanosheets for Oxygen Evolution Reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- Adhikari, S.; Eswar, N.K.; Sangita, S.; Sarkar, D.; Madras, G. Investigation of nano Ag-decorated SiC particles for photoelectrocatalytic dye degradation and bacterial inactivation. J. Photochem. Photobiol. A Chem. 2018, 357, 118–131. [Google Scholar] [CrossRef]
- Hao, J.-Y.; Wang, Y.-Y.; Tong, X.-L.; Jin, G.-Q.; Guo, X.-Y. Photocatalytic hydrogen production over modified SiC nanowires under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 15038–15044. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Ling, C.; Bai, X.; Ouyang, Y.; Du, A.; Wang, J. Single Molybdenum Atom Anchored on N-Doped Carbon as a Promising Electrocatalyst for Nitrogen Reduction into Ammonia at Ambient Conditions. J. Phys. Chem. C 2018, 122, 16842–16847. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Lyu, M.; Fan, S.; Liu, Q.; Zhang, W.; Zhi, Y.; Wang, C.; Xiao, C.; Wei, S.; et al. Low overpotential in vacancy-rich ultrathin CoSe2 nanosheets for water oxidation. J. Am. Chem. Soc. 2014, 136, 15670–15675. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef] [PubMed]









| Catalysts | Raw Material | Optimum Temperature (°C) | Pressure (MPa) | Space Velocity (mL·g−1·h−1) | CO Conversion (%) | CH4 Selectivity (%) | Space-Time Yield CH4 (mL·g−1·h−1) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 7%Ce-10%Ni/SiC | SiO2 | 600 | 1 | 60,000 | 95 | 85 | 41,182 | [28] |
| 10%Ni/VMT-SiO2 | Vermiculite (VMT) | 450 | 1.5 | 20,745 | 85.9 | 78 | 13,899 | [31] |
| 10%Ni/SiO2 | SiO2 | 420 | -- | 60,000 | 95.8 | 75.2 | 43,225 | [29] |
| 10%NiO/VMT-LDO | VMT-waste water | 400 | 1.5 | 20,000 | 87.88 | 89.97 | 15,813 | [9] |
| 10%Ni/SBA-15(ET) | Tetraethylorthosilicate | 400 | 0.3 | 15,000 | 100 | -- | -- | [30] |
| 10%NiO/Al2O3 | Al2O3 | 350 | 0.1 | 120,000 | 91.2 | 75 | 82,080 | [32] |
| 10%Ni/SF-SiC | Waste silica fume | 350 | 0.1 | 18,000 | 99.1 | 85.7 | 15,287 | This work |
| 20%NiO/Al2O3-SiC | SiO2 | 320 | 0.1 | 30,000 | 99 | 82 | 25,800 | [21] |
| Samples | SBET (m2/g) 1 | Vp (cm3/g) 2 | Vp (cm3/g) 2* | Dp (nm) 2 | Dp (nm) 2* | DNi (nm) 3 | DNi (nm) 4 |
|---|---|---|---|---|---|---|---|
| SF-SiO2 | 18.6 | 0.10 | 0.10 | 36.4 | 39.6 | -- | -- |
| Ni/SF-SiO2 | 21.0 | 0.13 | 0.13 | 34.1 | 36.9 | 12.6 | 15.6 |
| u-Ni/SF-SiO2 | 21.2 | 0.13 | 0.13 | 31.3 | 33.0 | 18.9 | 17.7 |
| SF-SiC | 45.2 | 0.20 | 0.20 | 19.9 | 19.1 | -- | -- |
| Ni/SF-SiC | 36.7 | 0.18 | 0.18 | 22.3 | 22.1 | 10.9 | 16.9 |
| u-Ni/SF-SiC | 42.2 | 0.18 | 0.18 | 21.3 | 20.9 | 12.3 | 16.9 |
| Samples | Reduction Temperature (°C) | Relative Content (%) | H2 Consumption (mmol/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1 | α2 | β | γ | α1 | α2 | β | γ | α1 | α2 | β | γ | |
| NiO/SF-SiO2 | 350.7 | 400.0 | 488.1 | -- | 32.9 | 40.5 | 26.6 | -- | 0.46 | 0.56 | 0.37 | -- |
| NiO/SF-SiC | 354.3 | 396.8 | 510.3 | 817.4 | 24.6 | 31.1 | 43.5 | 0.8 | 0.33 | 0.41 | 0.58 | 0.09 |
| Samples | Ni (%) | Ni0/Ni (%) | Ni2+/Ni (%) | Ni3+/Ni (%) | O (%) | Si (%) | C (%) |
|---|---|---|---|---|---|---|---|
| Ni/SF-SiO2 | 4.7 | 11.0 | 62.0 | 27.0 | 59.7 | 27.1 | 8.6 |
| Ni/SF-SiC | 5.2 | 11.2 | 57.8 | 31.0 | 44.2 | 30.5 | 20.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Q.; Zhai, X.; Yu, F.; Li, J.; Ren, X.; Zhang, H.; Zhu, M.; Dai, B.; Ge, G.; Zhang, J. Defect-Rich Nickel Nanoparticles Supported on SiC Derived from Silica Fume with Enhanced Catalytic Performance for CO Methanation. Catalysts 2019, 9, 295. https://doi.org/10.3390/catal9030295
Song Q, Zhai X, Yu F, Li J, Ren X, Zhang H, Zhu M, Dai B, Ge G, Zhang J. Defect-Rich Nickel Nanoparticles Supported on SiC Derived from Silica Fume with Enhanced Catalytic Performance for CO Methanation. Catalysts. 2019; 9(3):295. https://doi.org/10.3390/catal9030295
Chicago/Turabian StyleSong, Qi, Xingwu Zhai, Feng Yu, Jiangbing Li, Xin Ren, Haiyang Zhang, Mingyuan Zhu, Bin Dai, Guixian Ge, and Jinli Zhang. 2019. "Defect-Rich Nickel Nanoparticles Supported on SiC Derived from Silica Fume with Enhanced Catalytic Performance for CO Methanation" Catalysts 9, no. 3: 295. https://doi.org/10.3390/catal9030295