Catalytic Cracking of Biodiesel Waste Using Metal Supported SBA-15 Mesoporous Catalysts
Abstract
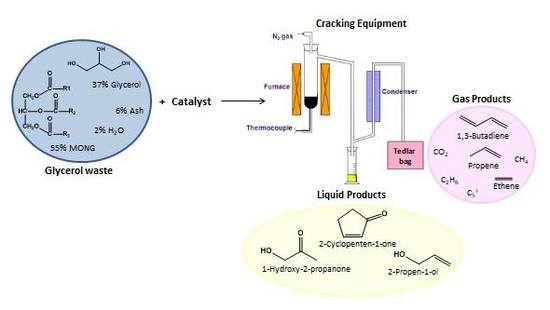
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Performance
2.2.1. Effect of the Starting Material
2.2.2. Effect of the Reaction Temperature without a Catalyst (Thermal Cracking)
2.2.3. Catalytic Cracking of Biodiesel Waste
2.2.4. Effect of the Amount of Catalyst
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zakaria, Z.Y.; Amin, N.A.S.; Linnekoski, J. A perspective on catalytic conversion of glycerol to olefins. Biomass Bioenergy 2013, 55, 370–385. [Google Scholar] [CrossRef]
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Shahirah, M.N.N.; Ayodele, B.V.; Gimbun, J.; Lam, S.S.; Cheng, C.K. Renewable syngas production from thermal cracking of glycerol over praseodymium-promoted Ni/Al2O3 catalyst. Appl. Therm. Eng. 2017, 112, 871–880. [Google Scholar] [CrossRef]
- Naik, D.V.; Singh, K.K.; Kumar, V.; Prasad, B.; Behera, B.; Bangwal, D.P.; Atheya, N.; Garg, M.O. Catalytic Cracking of Glycerol to Fine Chemicals over Equilibrium Fluid Catalytic Cracking Catalyst. Energy Procedia 2014, 54, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Geng, Z.; Zhang, M.; Yu, Y. Theoretical investigation on pyrolysis mechanism of glycerol. Fuel 2012, 93, 92–98. [Google Scholar] [CrossRef]
- Fernández, Y.; Arenillas, A.; Díez, M.A.; Pis, J.J.; Menéndez, J.A. Pyrolysis of glycerol over activated carbons for syngas production. J. Anal. Appl. Pyrolysis. 2009, 84, 145–150. [Google Scholar] [CrossRef]
- Fantozzi, F.; Frassoldati, A.; Bartocci, P.; Cinti, G.; Quagliarini, F.; Bidini, G.; Ranzi, E.M. An experimental and kinetic modeling study of glycerol pyrolysis. Appl. Energy 2016, 184, 68–76. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L.; Liu, H.; He, D. Influence of pretreatment on the catalytic performance of Ru/SBA-15 catalysts for glycerol hydrogenolysis. Chin. J. Catal. 2014, 35, 677–683. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Vila, F.; Mariscal, R.; Jiménez-López, A. Hydrogenolysis of glycerol to obtain 1,2-propanediol on Ce-promoted Ni/SBA-15 catalysts. Appl. Catal. B 2012, 117–118, 253–259. [Google Scholar] [CrossRef]
- Wu, Z.; Yan, H.; Ge, S.; Gao, J.; Dou, T.; Yongfeng, L.; Yip, C.K.A.; Zhang, M. MoO3 modified Ni2P/Al2O3 as an efficient catalyst for crude glycerol to propylene. Catal. Commun. 2017, 92, 80–85. [Google Scholar] [CrossRef]
- Kovacs, D.G.; Jackson, J.E.; Miller, D.J. Mechanistic investigation of glycerol hydrogenolysis. Rev. Roum. Chem. 2015, 60, 735–742. [Google Scholar]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.F.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J. Catal. 2008, 257, 163–171. [Google Scholar] [CrossRef]
- Zakaria, Z.Y.; Linnekoski, J.; Amin, N.A.S. Catalyst screening for conversion of glycerol to light olefins. Chem. Eng. J. 2012, 207–208, 803–813. [Google Scholar] [CrossRef]
- Neves, T.M.; Fernandes, J.O.; Liao, L.M.; da Silva, E.D.; da Rosa, C.A.; Mortola, V.B. Glycerol dehydration over micro- and mesoporous ZSM-5 synthesized from a one-step method. Microporous Mesoporous Mater. 2019, 275, 244–252. [Google Scholar] [CrossRef]
- Kraleva, E.; Atia, H. Keggin-type heteropolyacids supported on sol-gel oxides as catalysts for the dehydration of glycerol to acrolein. React. Kinet. Mech. Catal. 2019, 126, 103–117. [Google Scholar] [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V.D.B.C.; Likozar, B. Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification. Catal. Commun. 2017, 100, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Hu, J.; An, S.; Zhang, Q.; Guo, Y.; Song, D.; Shang, Q. Selective esterification of glycerol with acetic acid or lauric acid over rod-like carbon-based sulfonic acid functionalized ionic liquids. Fuel 2017, 207, 136–145. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Gómez-Siurana, A.; Marcilla, A.; Beltrán, M.; Berenguer, D.; Martínez-Castellanos, I.; Catalá, L.; Menargues, S. TGA/FTIR study of the MCM-41-catalytic pyrolysis of tobacco and tobacco–glycerol mixtures. Thermochim. Acta 2014, 587, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Shahirah, M.N.N.; Gimbun, J.; Ideris, A.; Khan, M.R.; Cheng, C.K. Catalytic pyrolysis of glycerol into syngas over ceria-promoted Ni/α-Al2O3 catalyst. Renew. Energy 2017, 107, 223–234. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L. Hydrogen production by glycerol steam reforming over SBA-15-supported nickel catalysts: Effect of alkaline earth promoters on activity and stability. Catal. Today 2014, 227, 198–206. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with Zr, Ce and La. J. Energy Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, J.M.; Santamaría González, J.; Moreno-Tost, R.; Maireles-Torres, P. WO3 supported on Zr doped mesoporous SBA-15 silica for glycerol dehydration to acrolein. Appl. Catal. A 2016, 516, 30–40. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Jin, Y. Catalytic dehydration of glycerol to acrolein over HPW supported on Cs+ modified SBA-15. Catal. Today 2014, 233, 127–132. [Google Scholar] [CrossRef]
- He, S.; Muizebelt, I.; Heeres, A.; Schenk, N.J.; Blees, R.; Heeres, H.J. Catalytic pyrolysis of crude glycerol over shaped ZSM-5/bentonite catalysts for bio-BTX synthesis. Appl. Catal. B 2018, 45, 45–55. [Google Scholar] [CrossRef]
- Arcanjo, M.R.A.; Silva, I.J.; Rodríguez-Castellón, E.; Infantes-Molina, A.; Vieira, R.S. Conversion of glycerol into lactic acid using Pd or Pt supported on carbon as catalyst. Catal. Today 2017, 279, 317–326. [Google Scholar] [CrossRef]
- Liu, R.; Lyu, S.; Wang, T. Sustainable production of acrolein from biodiesel-derived crude glycerol over H3PW12O40 supported on Cs-modified SBA-15. J. Ind. Eng. Chem. 2016, 37, 354–360. [Google Scholar] [CrossRef]
- de Sousa, F.F.; Oliveira, A.C.; Filho, J.M.; Pinheiro, G.S.; Giotto, M.; Barros, N.A.; Souza, H.S.A.; Oliveira, A.C. Metal oxides nanoparticles from complexes on SBA-15 for glycerol conversion. Chem. Eng. J. 2013, 228, 442–448. [Google Scholar] [CrossRef]
- Jiraroj, D.; Chaipurimat, A.; Kerdsa, N.; Hannongbua, S.; Tungasmita, D.N. Catalytic cracking of polypropylene using aluminosilicate catalysts. J. Anal. Appl. Pyrolysis. 2016, 120, 529–539. [Google Scholar] [CrossRef]
- Zeng, S.; Blanchard, J.; Breysse, M.; Shi, Y.; Shu, X.; Nie, H.; Li, D. Post-synthesis alumination of SBA-15 in aqueous solution: A versatile tool for the preparation of acidic Al-SBA-15 supports. Microporous Mesoporous Mater. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Fang, B.; Chaudhari, N.K.; Kim, M.-S.; Kim, J.H.; Yu, J.-S. Homogeneous Deposition of Platinum Nanoparticles on Carbon Black for Proton Exchange Membrane Fuel Cell. J. Am. Chem. Soc. 2009, 131, 15330–15338. [Google Scholar] [CrossRef]
- Velasquez, M.; Santamaria, A.; Batiot-Dupeyrat, C. Selective conversion of glycerol to hydroxyacetone in gas phase over La2CuO4 catalyst. Appl. Catal. B 2014, 160–161, 606–613. [Google Scholar] [CrossRef]
- Martinuzzi, I.; Azizi, Y.; Devaux, J.-F.; Tretjak, S.; Zahraa, O.; Leclerc, J.-P. Reaction mechanism for glycerol dehydration in the gas phase over a solid acid catalyst determined with on-line gas chromatography. Chem. Eng. Sci. 2014, 116, 118–127. [Google Scholar] [CrossRef]
- Liu, Y.; Tuysuz, H.; Jia, C.-J.; Schwickardi, M.; Rinaldi, R.; Lu, A.-H.; Schmidt, W.; Schuth, F. From glycerol to allyl alcohol: Iron oxide catalyzed dehydration and consecutive hydrogen transfer. Chem. Commun. 2010, 46, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Fredrickson, N.M.G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of MesoporousSilica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Hartmann, M.; Zhao, D.; Zhou, W.; Kevan, L. Alumination and Ion Exchange of Mesoporous SBA-15 Molecular Sieves. Chem. Mater. 1999, 11, 1621–1627. [Google Scholar] [CrossRef]
- Kanda, Y.; Aizawa, T.; Kobayashi, T.; Uemichi, Y.; Namba, S.; Sugioka, M. Preparation of highly active AlSBA-15-supported platinum catalyst for thiophene hydrodesulfurization. Appl. Catal. B 2007, 77, 117–124. [Google Scholar] [CrossRef] [Green Version]








| Catalyst | Total Specific Surface Area a (m2·g−1) | External Surface Area b (m2·g−1) | Pore size Distribution c (nm) | Pore Volume c (cm3·g−1) | d(100)d (nm) |
|---|---|---|---|---|---|
| SBA-15 | 925 | 51 | 9.23 | 1.24 | 9.69 |
| Al–SBA-15 | 469 | 49 | 9.23 | 0.90 | 9.31 |
| Pd–SBA-15 | 740 | 42 | 9.23 | 1.05 | 10.10 |
| Catalysts | Temp. (°C) | Catalyst Amount (wt%) | Starting Material | % Conversion | % Product Selectivity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Gas fraction | Liquid fraction | Distilled Liquid | Heavy Liquid | Residue | |||||
| Thermal cracking | 400 | - | Pure glycerol | 87.8 | 4.0 | 83.8 | 7.8 | 76.0 | 12.2 |
| Thermal cracking | 400 | - | Biodiesel waste | 63.5 | 14.5 | 49.0 | 22.4 | 26.6 | 36.5 |
| Thermal cracking | 650 | - | Biodiesel waste | 85.0 | 28.2 | 56.7 | 23.3 | 33.5 | 15.1 |
| Thermal cracking | 800 | - | Biodiesel waste | 86.3 | 27.3 | 59.0 | 23.8 | 35.2 | 13.7 |
| SBA-15 | 400 | 10 | Biodiesel waste | 66.5 | 15.9 | 50.6 | 15.1 | 35.5 | 42.5 |
| Al–SBA-15 | 400 | 10 | Pure glycerol | 89.6 | 5.7 | 83.9 | 9.0 | 74.9 | 10.4 |
| Al–SBA-15 | 400 | 10 | Biodiesel waste | 65.5 | 17.5 | 48.0 | 18.8 | 29.2 | 34.5 |
| Al–SBA-15 | 650 | 10 | Biodiesel waste | 86.8 | 25.6 | 61.2 | 25.8 | 35.4 | 13.2 |
| Pd–SBA-15 | 400 | 10 | Pure glycerol | 93.4 | 14.1 | 79.3 | 12.9 | 66.4 | 6.6 |
| Pd–SBA-15 | 400 | 10 | Biodiesel waste | 69.5 | 25.1 | 45.4 | 15.1 | 30.3 | 29.5 |
| Pd–SBA-15 | 650 | 5 | Biodiesel waste | 85.7 | 26.4 | 59.3 | 20.3 | 39.0 | 14.3 |
| Pd–SBA-15 | 650 | 10 | Biodiesel waste | 86.8 | 33.2 | 53.6 | 18.3 | 35.3 | 13.2 |
| Pd–SBA-15 | 650 | 15 | Biodiesel waste | 86.9 | 32.2 | 54.7 | 18.9 | 35.9 | 13.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiraroj, D.; Tongtooltush, T.; Panpranot, J.; Praserthdam, P.; Tungasmita, D.N. Catalytic Cracking of Biodiesel Waste Using Metal Supported SBA-15 Mesoporous Catalysts. Catalysts 2019, 9, 291. https://doi.org/10.3390/catal9030291
Jiraroj D, Tongtooltush T, Panpranot J, Praserthdam P, Tungasmita DN. Catalytic Cracking of Biodiesel Waste Using Metal Supported SBA-15 Mesoporous Catalysts. Catalysts. 2019; 9(3):291. https://doi.org/10.3390/catal9030291
Chicago/Turabian StyleJiraroj, Duangkamon, Tunyatorn Tongtooltush, Joongjai Panpranot, Piyasan Praserthdam, and Duangamol Nuntasri Tungasmita. 2019. "Catalytic Cracking of Biodiesel Waste Using Metal Supported SBA-15 Mesoporous Catalysts" Catalysts 9, no. 3: 291. https://doi.org/10.3390/catal9030291