Titanium-Dioxide-Based Visible-Light-Sensitive Photocatalysis: Mechanistic Insight and Applications
Abstract
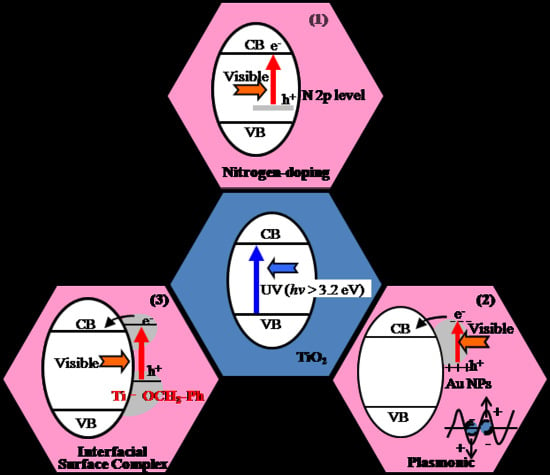
:1. Introduction
2. Nitrogen-doped TiO2 Photocatalysts
2.1. Synthesis of N-doped TiO2 Photocatalyst
2.1.1. Dry Processes
2.1.2. Wet Processes
2.2. N-states in N-doped TiO2
2.2.1. DFT Calculations
2.2.2. XPS Spectra
2.2.3. Optical Properties
2.2.4. Electron Paramagnetic Resonance (EPR) Spectra
2.2.5. Photo-Electrochemical Properties
2.3. Application to Photocatalytic Decomposition of Volatile Organic Compounds (VOC)
2.4. C3N4-Modified TiO2 Compared with N-doped TiO2
3. Plasmonic Au NPs Modified TiO2
3.1. What Is Localized Surface Plasmon Resonance (LSPR)?
3.2. Preparation and Characterization of Au–TiO2 Photocatalyst
3.2.1. Photodeposition (PD) Methods
3.2.2. Colloid Photodeposition Operated in the Presence of a Hole Scavenger (CPH)
3.2.3. Deposition Precipitation (DP) Method
3.2.4. Characterization of the Au–TiO2 Photocatalyst
3.3. Application of LSPR of Au–TiO2 to Several Photocatalytic Reactions
3.4. Application to a Photovoltaic Fuel Cell Operating under Visible Light Irradiation
3.5. Mechanisms of Charge Separation
4. Photo-Induced Interfacial Charge Transfer
4.1. Dye-Sensitized TiO2 Photocatalysis
4.2. Visible-Light-Responsive TiO2 Photocatalyst Modified by Phenolic Organic Compounds
4.3. Interfacial-Surface-Complex-Mediated Visible-Light-Sensitive TiO2 Photocatalysts
4.3.1. What Is the Origin of the Visible Light Response?
4.3.2. What Makes the High Selectivity for the Photocatalytic Reactions?
4.3.3. Reaction Mechanisms behind the Selective Photocatalytic Oxidation of Benzyl Alcohol
4.4. Photocatalytic Oxidation of Benzyl Amine into Imine
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| NPs | nanoparticles |
| ISC | interfacial surface complex |
| VOCs | volatile organic compounds |
| V.B. | valence band |
| C.B. | conduction band |
| XPS | X-ray photoelectron spectroscopy |
| EPR | electron paramagnetic resonance |
| UV-vis | Ultraviolet-visible |
| LSPR | localized surface plasmon resonance |
| PD | photodeposition |
| CPH | colloid photodeposition by hole scavenger |
| DP | deposition precipitation |
| TEM | transmittance electron microscope |
| JSC | short-circuit photocurrent |
| IPCE | incident photo to current efficiency |
| DFT | density functional theory |
| MLCT | metal to ligand charge transfer |
| FT-IR | Fourier transformed-infrared |
| KIE | kinetic isotope effect |
| LMCT | ligand to metal charge transfer |
References
- Honda, K.; Fijishima, A. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Kamat, P.V. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem. Rev. 1993, 93, 267–300. [Google Scholar]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar]
- Hoffman, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, A. Titanium dioxide photocatalysis. J. Photochem. Photobio. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 197, 2891–2959. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hirai, T. Selective organic transformations on titanium oxide-based photocatalysts. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 157–170. [Google Scholar] [CrossRef]
- Palmisano, G.; García-López, E.; Marcì, G.; Loddo, V.; Yurdakal, S.; Augugliaro, V.; Palmisano, L. Advances in selective conversions by heterogeneous photocatalysis. Chem. Commun. 2010, 46, 7074–7089. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for: Photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visiblelight-sensitive photocatalyst: Designs, developments, and prospects. Chem Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as Functional Building Blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity Enhancement in Heterogeneous Photocatalytic Transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Yamashita, H.; Mori, K.; Kuwahara, Y.; Kamegawa, T.; Wen, M.; Verma, P.; Che, M. Single-site and nano-confined photocatalysts designed in porous materials for environmental uses and solar fuels. Chem. Soc. Rev. 2018, 47, 8072–8096. [Google Scholar] [CrossRef]
- Ahmed, A.Y.; Kandiel, T.A.; Oekermann, T.; Bahnemann, D. Photocatalytic Activities of Different Well-defined Single Crystal TiO2Surfaces: Anatase versus Rutile. J. Phys. Chem. Lett. 2011, 2, 2461–2465. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.V.; Hisanaga, T. Effect of Crystallinity of TiO2 on Its Photo-catalytic Action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile? - Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043–4050. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.J.; Ying, Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Anpo, M.; Ichihashi, Y.; Takeuchi, M.; Yamashita, H. Design of unique titanium oxide photocatalysts by an advanced metal ion-implantation method and photocatalytic reactions under visible light irradiation. Res. Chem. Intermed. 1998, 24, 143–149. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Shin, C.; Bugli, G.; Djega-Mariadassou, G. Preparation and characterization of titanium oxynitrides with high specific surface areas. J. Solid State Chem. 1991, 95, 145–155. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, Y.; Liu, S. An efficient two-step technique for nitrogen-doped titanium dioxide synthesizing: Visible-light-induced photodecomposition of methylene blue. J. Phys. Chem. C 2007, 111, 1010–1014. [Google Scholar] [CrossRef]
- Higashimoto, S.; Azuma, M. Photo-induced charging effect and electron transfer to the redox species on nitrogen-doped TiO2 under visible light irradiation. Appl. Catal. B Environ. 2009, 89, 557–562. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y. The Photocatalytic Property of Nitrogen-Doped TiO2 Nanoball Film. Int. J. Photoenergy 2013. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Goralski, E.G.; Walck, S.D.; Yates, J.T., Jr. Photochemical Activity of Nitrogen-Doped Rutile TiO2(110) in Visible Light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Higashimoto, S.; Ushiroda, Y.; Azuma, M.; Ohue, H. Synthesis, characterization and photocatalytic activity of N-doped TiO2 modified by platinum chloride. Catal. Today 2008, 132, 165–169. [Google Scholar] [CrossRef]
- Higashimoto, S.; Ushiroda, Y.; Azuma, M. Mechanism for enhancement of visible light response on nitrogen-doped TiO2 by modification with vanadium species. J. Nanosci. Nanotechnol. 2010, 10, 246–251. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for Visible Light Responses in Anodic Photocurrents at N-Doped TiO2 Film Electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Tang, J.; Cowan, A.J.; Durrant, J.R.; Klug, D.R. Mechanism of O2 production from water splitting: Nature of charge carriers in nitrogen doped nanocrystalline TiO2 films and factors limiting O2 production. J. Phys. Chem. C 2011, 115, 3143–3150. [Google Scholar] [CrossRef]
- Higashimoto, S.; Tanihata, W.; Nakagawa, Y.; Azuma, M.; Ohue, H.; Sakata, Y. Effective photocatalytic decomposition of VOC under visible-light irradiation on N-doped TiO2 modified by vanadium species. Appl. Catal. A Gen. 2008, 340, 98–104. [Google Scholar] [CrossRef]
- Morikawa, T.; Ohwaki, T.; Suzuki, K.; Moribe, S.; Tero-Kubota, S. Visible-light-induced photocatalytic oxidation of carboxylic acids and aldehydes over N-doped TiO2 loaded with Fe, Cu or Pt. Appl. Catal. B Environ. 2008, 83, 56–62. [Google Scholar] [CrossRef]
- Sreethawong, T.; Laehsalee, S.; Chavadej, S. Use of Pt/N-doped mesoporous-assembled nanocrystalline TiO2 for photocatalytic H2 production under visible light irradiation. Catal. Commun. 2009, 10, 538–543. [Google Scholar] [CrossRef]
- Dolat, D.; Quici, N.; Kusiak-Nejman, E.; Morawski, A.W.; Puma, G.L. One-step, hydrothermal synthesis of nitrogen, carbon co-doped titanium dioxide (N,C-TiO2) photocatalysts. Effect of alcohol degree and chain length as carbon dopant precursors on photocatalytic activity and catalyst deactivation. Appl. Catal. B Environ. 2012, 115, 81–89. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Visible light activity of Ag-loaded and guanidine nitrate-doped nano-TiO2: Degradation of dichlorophenol and antibacterial properties. RSC Adv. 2012, 2, 1533–1539. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and Characterization of Nitrogen-Doped TiO2Nanophotocatalyst with High Visible Light Activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping. J. Catal. 2008, 260, 128–133. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. On the Mechanism of Urea–Induced Titania Modification. Chem. Eur. J. 2010, 16, 261–269. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 2012, 14, 16745–16752. [Google Scholar] [CrossRef]
- Han, C.; Wang, Y.; Lei, Y.; Wang, B.; Wu, N.; Shi, Q.; Li, Q. In situ synthesis of graphitic-C3N4 nanosheet hybridized N-doped TiO2 nanofibers for efficient photocatalytic H2 production and degradation. Nano Res. 2015, 8, 1199–1209. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H. TiO2-g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloy. Comp. 2010, 509, L26–L29. [Google Scholar] [CrossRef]
- Higashimoto, S.; Hikita, K.; Azuma, M.; Yamamoto, M.; Takahashi, M.; Sakata, Y.; Matsuoka, M.; Kobayashi, H. Visible Light-Induced Photocatalysis on Carbon Nitride Deposited Titanium Dioxide: Hydrogen Production from Sacrificial Aqueous Solutions. Chin. J. Chem. 2017, 35, 165–172. [Google Scholar] [CrossRef]
- Zhang, Q.; Gangadharan, D.T.; Liu, Y.; Xu, Z.; Chaker, M.; Ma, D. Recent advancements in plasmon-enhanced visible light-driven water splitting. J. Materiomics 2017, 3, 33–50. [Google Scholar] [CrossRef]
- Ohko, Y.; Tatsuma, T.; Fujii, T.; Naoi, K.; Niwa, C.; Kubota, Y.; Fujishima, A. Multicolour photochromism of TiO2 films loaded with silver nanoparticles. Nat. Mater. 2003, 2, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tatsuma, T. Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem. Commun. 2004, 0, 1810–1811. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 0, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Kolinko, P.A.; Selishchev, D.S.; Kozlov, D.V. Visible Light Photocatalytic Oxidation of Ethanol Vapor on Titanium Dioxide Modified with Noble Metals. Theor. Exp. Chem. 2015, 51, 96–103. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Tanaka, A.; Ogino, A.; Iwaki, M.; Hashimoto, K.; Ohnuma, A.; Amano, F.; Ohtani, B.; Kominami, H. Gold–Titanium(IV) Oxide Plasmonic Photocatalysts Prepared by a Colloid-Photodeposition Method: Correlation Between Physical Properties and Photocatalytic Activities. Langmuir 2012, 28, 13105–13111. [Google Scholar] [CrossRef]
- Silva, C.G.; Juarez, R.; Marino, T.; Molinari, R.; Garcia, H. Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Zanella, R.; Delannoy, L.; Louis, C. Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition–precipitation with NaOH and urea. Appl. Catal. A Gen. 2005, 291, 62–72. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 with Metal Cocatalysts Exhibiting Strong Surface Plasmon Resonance Effective for Photoinduced Hydrogen Formation under Irradiation of Visible Light. ACS Catal. 2013, 3, 79–85. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakanishi, K.; Hamada, R.; Hashimoto, K.; Kominami, H. Simultaneous and Stoichiometric Water Oxidation and Cr(VI) Reduction in Aqueous Suspensions of Functionalized Plasmonic Photocatalyst Au/TiO2–Pt under Irradiation of Green Light. ACS Catal. 2013, 3, 1886–1891. [Google Scholar] [CrossRef]
- Tanaka, A.; Nishino, Y.; Sakaguchi, S.; Yoshikawa, T.; Imamura, K.; Hashimoto, K.; Kominami, H. Functionalization of a plasmonic Au/TiO2 photocatalyst with an Ag co-catalyst for quantitative reduction of nitrobenzene to aniline in 2-propanol suspensions under irradiation of visible light. Chem. Commun. 2013, 49, 2551–2553. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hashimoto, K.; Kominami, H. A very simple method for the preparation of Au/TiO2 plasmonic photocatalysts working under irradiation of visible light in the range of 600–700 nm. Chem. Commun. 2017, 53, 4759–4762. [Google Scholar] [CrossRef]
- Naya, S.; Teranishi, M.; Isobe, T.; Tada, H. Light wavelength-switchable photocatalytic reaction by gold nanoparticle-loaded titanium(IV) dioxide. Chem. Commun. 2010, 46, 815–817. [Google Scholar] [CrossRef]
- Naya, S.; Kimura, K.; Tada, H. One-Step Selective Aerobic Oxidation of Amines to Imines by Gold Nanoparticle-Loaded Rutile Titanium(IV) Oxide Plasmon Photocatalyst. ACS Catal. 2013, 3, 10–13. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Facile in situ synthesis of visible-light plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag) and evaluation of their photocatalytic oxidation of benzene to phenol. J. Mater. Chem. 2011, 21, 9079–9087. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation from organic and inorganic compounds under irradiation of visible light. Catal. Sci. Technol. 2012, 2, 907–909. [Google Scholar] [CrossRef]
- Tanaka, A.; Teramura, K.; Hosokawa, S.; Kominami, H.; Tanaka, T. Visible light-induced water splitting in an aqueous suspension of a plasmonic Au/TiO2 photocatalyst with metal co-catalysts. Chem. Sci. 2017, 8, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast Plasmon-Induced Electron Transfer from Gold Nanodots into TiO2 Nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- Borgarello, E.; Kiwi, J.; Pelizzetti, E.; Visca, M.; Grätzel, M. Photochemical cleavage of water by photocatalysis. Nature 1981, 289, 158–160. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Hua, X.; Dahlgren, R.L. Photochemistry of Ru(bpy)2(dcbpy)2+ on A12O3 and TiO2 surfaces. an insight into the mechanism of photosensitization. J. Phys. Chem. 1995, 99, 10883–10889. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Macyk, W.; Burgeth, G.; Kisch, H. Photoelectrochemical properties of platinum(IV) chloride surface modified TiO2. Photochem. Photobiol. Sci. 2003, 2, 322–328. [Google Scholar] [CrossRef]
- Higashimoto, S.; Nishi, T.; Yasukawa, M.; Azuma, M.; Sakata, Y.; Kobayashi, H. Photocatalysis of titanium dioxide modified by interfacial surface complexes (ISC) with different substituted groups. J. Catal. 2015, 329, 286–290. [Google Scholar] [CrossRef]
- Ikeda, S.; Abe, C.; Torimoto, T.; Ohtani, B. Photochemical hydrogen evolution from aqueous triethanolamine solutions sensitized by binaphthol-modified titanium(IV) oxide under visible-light irradiation. J. Photochem. Photobiol. A Chem. 2003, 160, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Kamegawa, T.; Seto, H.; Matsuura, S.; Yamashita, H. Preparation of hydroxynaphthalene modified TiO2 via formation of surface complexes and their applications in the photocatalytic reduction of nitrobenzene under visible-light irradiation. ACS Appl. Mater. Interfaces 2012, 4, 6635–6639. [Google Scholar] [CrossRef]
- Kim, S.; Choi, W. Visible-light-induced photocatalytic degradation of 4-chlorophenol and phenolic compounds in aqueous suspension of pure titania: Demonstrating the existence of a surface-complex-mediated path. J. Phys. Chem. B 2005, 109, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective photocatalytic oxidation of benzyl alcohol and its derivatives into corresponding aldehydes by molecular oxygen on titanium dioxide under visible light irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Higashimoto, S.; Okada, K.; Morisugi, T.; Azuma, M.; Ohue, H.; Kim, T.-H.; Matsuoka, M.; Anpo, M. Effect of surface treatment on the selective photocatalytic oxidation of benzyl alcohol infrared study of hydroxy groups on coordinative defect sites. Top. Catal. 2010, 53, 578–583. [Google Scholar] [CrossRef]
- Higashimoto, S.; Okada, K.; Azuma, M.; Ohue, H.; Terai, T.; Sakata, Y. Characteristics of the charge transfer surface complex on titanium(IV) dioxide for the visible light induced chemoselective oxidation of benzyl alcohol. RSC Adv. 2012, 2, 669–676. [Google Scholar] [CrossRef]
- Higashimoto, S.; Suetsugu, N.; Azuma, M.; Ohue, H.; Sakata, Y. Efficient and selective oxidation of benzylic alcohol by O2 into corresponding aldehydes on a TiO2 photocatalyst under visible light irradiation: Effect of phenyl-ring substitution on the photocatalytic activity. J. Catal. 2010, 274, 76–83. [Google Scholar] [CrossRef]
- Higashimoto, S.; Shirai, R.; Osano, Y.; Azuma, M.; Ohue, H.; Sakata, Y.; Kobayashi, H. Influence of metal ions on the photocatalytic activity: Selective oxidation of benzyl alcohol on iron (III) ion-modified TiO2, using visible light. J. Catal. 2014, 311, 137–143. [Google Scholar] [CrossRef]
- Kobayashi, H.; Higashimoto, S. DFT study on the reaction mechanisms behind the catalytic oxidation of benzyl alcohol into benzaldehyde by O2 over anatase TiO2 surfaces with hydroxyl groups: Role of visible-light irradiation. Appl. Catal. B Environ. 2015, 170, 135–143. [Google Scholar] [CrossRef]
- Li, R.; Kobayashi, H.; Guo, J.; Fan, J. Visible-light induced high-yielding benzyl alcohol-to benzaldehyde transformation over mesoporous crystalline TiO2: A self-adjustable photooxidation system with controllable hole-generation. J. Phys. Chem. C 2011, 115, 23408–23416. [Google Scholar] [CrossRef]
- Lang, X.; Ma, W.; Zhao, Y.; Chen, C.; Ji, H.; Zhao, J. Visible-light-induced selective catalytic aerobic oxidation of amines into imines on TiO2. Chem. Eur. J. 2012, 18, 2624–2631. [Google Scholar] [CrossRef]
- Higashimoto, S.; Hatada, Y.; Ishikawa, R.; Azuma, M.; Sakata, Y.; Kobayashi, H. Selective Photocatalytic Oxidation of Benzyl Amine by O2 into N-Benzylidenebenzylamine on TiO2 Using Visible Light. Curr. Org. Chem. 2013, 17, 2374–2381. [Google Scholar] [CrossRef]




















| Entry | Reactant Molecules | Yields of CO2/μmol | |
|---|---|---|---|
| N-doped TiO2 | VCl3/N-doped TiO2 | ||
| 1 | methanol a | 0.2 | 1.1 |
| 2 | ethanol a | 0.3 | 0.5 |
| 3 | formaldehyde a | 4.6 | 21.6 |
| 4 | acetaldehyde a | 4.1 | 35.0 |
| 5 | formic acid a | 0.7 | 4.8 |
| 6 | acetic acid a | 1.2 | 17.0 |
| 7 | acetone a | 0.7 | 11.4 |
| 8 | ethyl acetate a | 1.3 | 10.6 |
| 9 | dichloromethane b | 2.4 | 4.1 |
| 10 | trichloromethane b | 1.5 | 4.1 |
| 11 | 1, 1-dichloroethane b | 0.7 | 4.8 |
| 12 | trans-1, 2-dichloroethylene b | 1.0 | 5.7 |
| Entry | Au Deposition Methods | Particle Sizes/nm | Top Peak/nm | Ref. |
|---|---|---|---|---|
| 1 | PD | ~10–60 | ~530–610 | [56,57,58] |
| 2 | CPH | ~12–14 | ~550–560 | [60,63,64,65,66] |
| 13 | ~550–620 | [67] | ||
| 3 | DP | ~2–6 | ~550–560 | [61] |
| < 5 | 550 | [68,69,70] |
| Entry | Photocatalytic Reactions | Au Deposition Methods | References |
|---|---|---|---|
| 1 | oxidations of 2-propanol and ethanol oxidation of formic acid | PD CPH | [56,57,58] [60] |
| 2 | oxidation of thiol to disulfide | DP | [67] |
| oxidation of amine to imine | DP | [68] | |
| oxidation of aromatic alcohol to aldehyde | CPH | [66] | |
| DP | [69] | ||
| oxidation of benzene to phenol | PD | [70] | |
| 3 | H2 formation from alcohols | CPH | [63,71] |
| water splitting into H2 and O2 | DP CPH | [61] [64,72] | |
| 4 | reduction of nitrobenzene to aniline | CPH | [65] |

| Entry | R1 | R2 | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| 1 | H | H | > 99 | > 99 |
| 2 | H | C(CH3)3 | > 99 | > 99 |
| 3 | H | OCH3 | > 99 | > 99 |
| 4 | H | CH3 | > 99 | > 99 |
| 5 | H | Cl | > 99 | > 99 |
| 6 | H | NO2 | > 99 | > 99 |
| 7 | H | CF3 | > 99 | > 99 |
| 8 | CH3 | H | > 99 | > 99 |
| 9 | H | OH | > 85 | 23 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashimoto, S. Titanium-Dioxide-Based Visible-Light-Sensitive Photocatalysis: Mechanistic Insight and Applications. Catalysts 2019, 9, 201. https://doi.org/10.3390/catal9020201
Higashimoto S. Titanium-Dioxide-Based Visible-Light-Sensitive Photocatalysis: Mechanistic Insight and Applications. Catalysts. 2019; 9(2):201. https://doi.org/10.3390/catal9020201
Chicago/Turabian StyleHigashimoto, Shinya. 2019. "Titanium-Dioxide-Based Visible-Light-Sensitive Photocatalysis: Mechanistic Insight and Applications" Catalysts 9, no. 2: 201. https://doi.org/10.3390/catal9020201