Design and Characterization of a Novel Artificial Peroxidase
Abstract
:1. Introduction
2. Results and Discussion
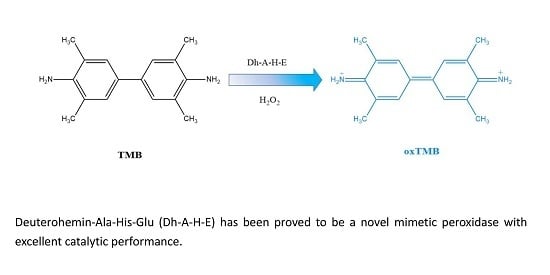
2.1. The Peroxidase Activity of Deuterohemin–Ala–His–Glu (Dh–A–H–E)
2.2. Optimizing the Reaction Conditions
2.3. Kinetics
2.4. Catalytic Mechanism
2.5. Stability of Dh–A–H–E
2.6. Application of Dh–A–H–E for H2O2 and Glucose Detection
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Dh–A–H–E
3.3. Standard Peroxidase Activity Assay of Dh–A–H–E
3.4. Optimizing the Reaction Conditions
3.5. Kinetics of Dh–A–H–E
3.6. Stability of Dh–A–H–E
3.7. Detection of the Trace Concentration of H2O2 and Glucose Using Dh–A–H–E
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, X.; Han, Y.; Jiang, Z.; Chen, D. Synthesis of Fe3O4–Au nanocomposites with enhanced peroxidase–like activity. Eur. J. Inorg. Chem. 2013, 1, 109–114. [Google Scholar] [CrossRef]
- Wang, M.; Bao, W.; Wang, J. A green approach to the synthesis of novel “Desert rose stone”—Like nanobiocatalytic system with excellent enzyme activity and stability. Sci. Rep. 2014, 4, 6606. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takahashi, H.; Miyake, Y. Convenient synthesis of neurotrophic americanol A and isoamericanol A by HRP catalyzed oxidative coupling of caffeic acid. Tetrahedron Lett. 1999, 40, 3185–3186. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, M.; Lin, Y. Specifically and reversibly immobilizing proteins/enzymes to nitriolotriacetic–acid–modified mesoporous silicas through histidine tags for purification or catalysis. Chem. Eur. J. 2011, 17, 13059–13067. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Nakano, M.; Kimura, T. On the mechanism of the peroxidase–catalyzed oxygen–transfer reaction. Biochemistry 1987, 26, 5019–5022. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. Co3O4 nanoparticles as an efficient catalase mimic: Properties, mechanism and its electrocatalytic sensing application for hydrogen peroxide. J. Mol. Catal. A Chem. 2013, 378, 30–37. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Guo, Z.; Hu, Z.; Xue, Z.; Lu, X. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens. Bioelectron. 2017, 87, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, D.; Long, Y.; Xie, Z.; Zheng, H. Intrinsic of peroxidase–like activity of ficin. Sci. Rep. 2017, 7, 43141. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Y.; Yang, Y.; Li, M.; Long, Y.; Huang, Y.; Zheng, H. Fluorescein as an artificial enzyme to mimic peroxidase. Chem. Commun. 2016, 52, 13912–13915. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, K.; Yu, Z.; Ding, J.; Hu, Q.; Liu, Q.; Sun, H.; Wen, D.; Liu, Q.; Kong, J. The peroxidase–like catalytic activity of ferrocene and its application in the biomimetic synthesis of microsphere polyaniline. New J. Chem. 2018, 42, 13536–13540. [Google Scholar] [CrossRef]
- Xia, X.; Long, Y.; Wang, J. Glucose oxidase–functionalized fluorescent gold nanoclusters as probes for glucose. Anal. Chim. Acta 2013, 772, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ghavidel Hajiagha, N.; Mahmoudi, A.; Sazegar, M.R.; Pouramini, M.M. Synthesis of cobalt–modified MSN as a model enzyme: Evaluation of the peroxidatic performance. Microporous Mesoporous Mater. 2019, 274, 43–53. [Google Scholar] [CrossRef]
- Kalaivani, G.; Sivanesan, A.; Kannan, A.; Sevvel, R. Generating monomeric 5–coordinated microperoxidase–11 using carboxylic acid functionalized silver nanoparticles: A surface–enhanced resonance Raman scattering analysis. Colloids Surf. B 2016, 146, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W. The broad diversity of heme–protein cross–links: An overview. Biochim. Biophys. Acta 2015, 1584, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Leboffe, L.; Santucci, R.; Coletta, M. Ferric microperoxidase–11 catalyzes peroxynitrite isomerization. J. Inorg. Biochem. 2015, 144, 56–61. [Google Scholar] [CrossRef]
- Guan, S.; Li, P.; Luo, J. A deuterohemin peptide extends lifespan and increases stress resistance in Caenorhabditis elegans. Free Radic. Res. 2010, 44, 813–820. [Google Scholar] [CrossRef]
- Casella, L.; Gullotti, M.; Monzani, E. Biomimetic oxidation catalysis by iron (III) deuteroporpbyrin carrying a deca–L–alanine peptide chain. Rend. Lincei 1991, 2, 201–212. [Google Scholar] [CrossRef]
- Casella, L.; Monzani, E.; Fantucci, P. Axial imidazole distortion effects on the catalytic and binding properties of chelated deuterohemin complexes. Inorg. Chem. 1996, 35, 439–444. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Zhao, M. 3D flower–like ferrous(II) phosphate nanostructures as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose at nanomolar level. Talanta 2018, 182, 230–240. [Google Scholar] [CrossRef]
- Pang, Y.; Huang, Z.; Yang, Y.; Long, Y.; Zheng, H. Colorimetric detection of glucose based on ficin with peroxidase–like activity. Spectrochim. Acta Part A 2018, 189, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Z.; Wen, F.; Tan, J.; Peng, T.; Luo, B.; Wang, H.; Yin, S. A flower–like MoS2–decorated MgFe2O4 nanocomposite: Mimicking peroxidase and colorimetric detection of H2O2 and glucose. Sens. Actuators B 2018, 275, 155–162. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, K.; Li, H.; Chen, W.; Fu, M.; Yue, K.; Zhu, X.; Liu, Q. Glutathione detection based on peroxidase–like activity of Co3O4–Montmorillonite nanocomposites. Sens. Actuators B 2018, 273, 1635–1639. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, L.; Yu, Y.; Long, Y.; Zheng, H. Acidic amino acids: A new–type of enzyme mimics with application to biosensing and evaluating of antioxidant behavior. Spectrochim. Acta Part A 2018, 201, 367–375. [Google Scholar] [CrossRef]
- Savic, S.; Vojinovic, K.; Milenkovic, S.; Smelcerovic, A.; Lamshoeft, M.; Petronijevic, Z. Enzymatic oxidation of rutin by horseradish peroxidase: Kinetic mechanism and identification of a dimeric product by LC–Orbitrap mass spectrometry. Food Chem. 2013, 141, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, T.; Chen, W.; Song, Y. Co4N Nanowires: Noble–Metal–Free Peroxidase Mimetic with Excellent Salt– and Temperature–Resistant Abilities. ACS Appl. Mater. Interfaces 2017, 9, 29881–29888. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shang, L.; Guo, S.; Fang, Y.; Wen, D.; Wang, L.; Yin, J.; Dong, S. Biomolecule–stabilized Au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens. Bioelectron. 2011, 26, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, H.; Rahman, Z.U.; Su, L.; Chen, X.; Hu, J.; Chen X., G. Graphene Oxide–Fe3O4 Magnetic Nanocomposites with peroxidase–Like Activity for colorimetric detection of glucose. Nanoscale 2012, 4, 3969–3976. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Shi, Y.; Yang, Y.; Zheng, H.; Long, Y. Dichlorofluorescein as a peroxidase mimic and its application to glucose detection. New J. Chem. 2017, 41, 7578–7582. [Google Scholar] [CrossRef]
- Malvi, B.; Panda, C.; Dhar, B.B.; Gupta, S.S. One pot glucose detection by [FeIII(biuret–amide)] immobilized on mesoporous silica nanoparticles: An efficient HRP mimic. Chem. Commun. 2012, 48, 5289–5291. [Google Scholar] [CrossRef]







| Catalyst | Substrate (TMB) | Substrate (H2O2) | ||||
|---|---|---|---|---|---|---|
| Km (mM) | Kcat (s−1) | Kcat/Km (s−1·M−1) | Km (mM) | Kcat (s−1) | Kcat/Km (s−1·M−1) | |
| Dh–A–H–E | 0.20 | 2.10 | 1.05 × 103 | 0.27 | 2.37 | 8.78 × 102 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Xu, J.; Zhao, Z.; Li, H.; Wang, K.; Wang, Z.; Wang, L. Design and Characterization of a Novel Artificial Peroxidase. Catalysts 2019, 9, 168. https://doi.org/10.3390/catal9020168
Yuan Y, Xu J, Zhao Z, Li H, Wang K, Wang Z, Wang L. Design and Characterization of a Novel Artificial Peroxidase. Catalysts. 2019; 9(2):168. https://doi.org/10.3390/catal9020168
Chicago/Turabian StyleYuan, Ye, Jia Xu, Zhenyu Zhao, Hui Li, Kai Wang, Zhi Wang, and Liping Wang. 2019. "Design and Characterization of a Novel Artificial Peroxidase" Catalysts 9, no. 2: 168. https://doi.org/10.3390/catal9020168