Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
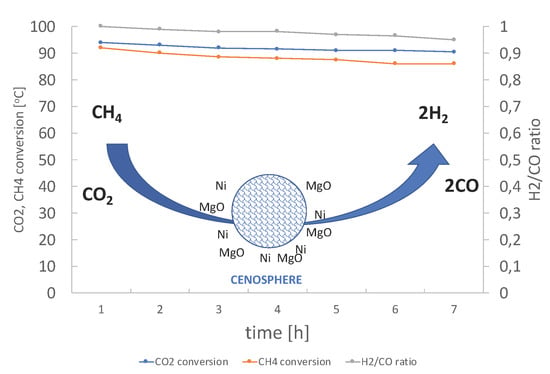
2.2. DRM Catalytic Tests
2.3. Stability Test
2.4. Post-Test Characterization of the Catalysts
3. Materials and Methods
3.1. Ni/Mg Cenosphere Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lecomte, T.; Ferrería De La Fuente, F.J.; Neuwahl, F.; Canova, M.; Pinasseau, A.; Jankov, I.; Brinkmann, T.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Large Combustion Plants-Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-74303-0. [Google Scholar]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Acar, I.; Atalay, M.U. Recovery potentials of cenospheres from bituminous coal fly ashes. Fuel 2016, 180, 97–105. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Costa, R.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Chandane, V.S.; Rathod, A.P.; Wasewar, K.L.; Sonawane, S.S. Efficient cenosphere supported catalyst for the esterification of n-octanol with acetic acid. C. R. Chim. 2017, 20, 818–826. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Mikhaylova, O.A.; Anshits, A.G. Composition and Morphology of Fly Ash Cenospheres Produced from the Combustion of Kuznetsk Coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Samojeden, B.; Drużkowska, J.; Duraczyńska, D.; Poddębniak, M.; Motak, M. Use of iron and copper-promoted cenospheres as catalysts in the selective catalytic reduction of nitrogen(II) oxide with ammonia. Przem. Chem. 2019, 1, 55–59. [Google Scholar]
- Zhang, J.; Wang, B.; Cui, H.; Li, C.; Zhai, J.; Li, Q. Synthesis of CeO2/fly ash cenospheres composites as novel photocatalysts by modified pyrolysis process. J. Rare Earths 2014, 32, 1120–1125. [Google Scholar] [CrossRef]
- Hosseini Asl, S.M.; Ghadi, A.; Sharifzadeh Baei, M.; Javadian, H.; Maghsudi, M.; Kazemian, H. Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: A review. Fuel 2018, 217, 320–342. [Google Scholar] [CrossRef]
- Samojeden, B. The current and future trends in chemical CO2 utilization. In Contemporary Problems of Power Engineering and Environmental Protection; Pikoń, K., Czarnowska, L., Eds.; Silesian University of Technology: Gliwice, Poland, 2018; pp. 215–226. ISBN 978-83-950087-1-9. [Google Scholar]
- Xu, L.; Song, H.; Chou, L. Ordered mesoporous MgO–Al2O3 composite oxides supported Ni based catalysts for CO2 reforming of CH4: Effects of basic modifier and mesopore structure. Int. J. Hydrog. Energy 2013, 38, 7307–7325. [Google Scholar] [CrossRef]
- Jeong, M.; Nunotani, N.; Imanaka, N. Relationship between the conductivities of CeO2-ZrO2-MOx (M = Bi, Ca, Sn, Ni, Fe) solid solutions and catalytic activities during methane oxidation. Bull. Chem. Soc. Jpn. 2018, 91, 158–164. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.F.; Chen, X.L.; Wang, A.J.; Han, D.M.; Wang, Z.G.; Feng, J.J. Facile solvothermal synthesis of Pt71Co29 lamellar nanoflowers as an efficient catalyst for oxygen reduction and methanol oxidation reactions. J. Colloid Interface Sci. 2019, 536, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, M.; Murakami, K.; Sekine, Y. Low-temperature heterogeneous catalytic reaction by surface protonics. Bull. Chem. Soc. Jpn. 2019, 92, 1785–1792. [Google Scholar] [CrossRef] [Green Version]
- Mota, F.M.; Kim, D.H. From CO2 methanation to ambitious long-chain hydrocarbons: Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Albarazi, A.; Beaunier, P.; Da Costa, P. Hydrogen and syngas production by methane dry reforming on SBA-15 supported nickel catalysts: On the effect of promotion by Ce0.75Zr0.25O2 mixed oxide. Int. J. Hydrog. Energy 2013, 38, 127–139. [Google Scholar] [CrossRef]
- Duan, Y.; Shang, R.; Zhong, X.; Xie, W.; Wang, X.; Huang, L. In-situ synthesis of NiMo2C/Al2O3 catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2016, 41, 21955–21964. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Da Costa, P. Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 23500–23507. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- BASF and Linde Successfully Complete Pilot Project at National Carbon Capture Center in Wilsonville, Alabama. Available online: https://www.linde-gaz.pl/pl/news_and_media/press_releases/news_20160719.html (accessed on 6 December 2019).
- Şener, A.N.; Günay, M.E.; Leba, A.; Yıldırım, R. Statistical review of dry reforming of methane literature using decision tree and artificial neural network analysis. Catalysis Today 2018, 299, 289–302. [Google Scholar]
- Dȩbek, R.; Wierzbicki, D.; Motak, M.; Galvez, M.E.; Da Costa, P.; Azzolina-Jury, F. Operando FT-IR study on basicity improvement of Ni(Mg,Al)O hydrotalcite-derived catalysts promoted by glow plasma discharge. Plasma Sci. Technol. 2019, 21, 045503. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrog. Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering 2018, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.; Da Costa, P. A Short Review on the Catalytic Activity of Hydrotalcite-Derived Materials for Dry Reforming of Methane. Catalysts 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Dębek, R.; Motak, M.; Galvez, M.E.; Da Costa, P.; Grzybek, T. Catalytic activity of hydrotalcite-derived catalysts in the dry reforming of methane: On the effect of Ce promotion and feed gas composition. React. Kinet. Mech. Catal. 2017, 121, 185–208. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Bel Hadjltaief, H.; Benzina, M.; Gálvez, M.E.; Da Costa, P. Natural clay based nickel catalysts for dry reforming of methane: On the effect of support promotion (La, Al, Mn). Int. J. Hydrog. Energy 2018, 1–10. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Molina, R.; Poncelet, G. α-Alumina-Supported Nickel Catalysts Prepared from Nickel Acetylacetonate: A TPR Study. J. Catal. 1998, 173, 257–267. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Duraczyńska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J. CO2 Util. 2018, 27, 247–258. [Google Scholar] [CrossRef]
- Liu, H.; Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Da Costa, P.; Galvez, M.E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16. [Google Scholar] [CrossRef]
- Titus, J.; Goepel, M.; Schunk, S.A.; Wilde, N.; Gläser, R. The role of acid/base properties in Ni/MgO-ZrO2–based catalysts for dry reforming of methane. Catal. Commun. 2017, 100, 76–80. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Syngas production from dry methane reforming over yttrium-promoted nickel-KIT-6 catalysts. Int. J. Hydrog. Energy 2019, 4, 274–286. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, G.; Li, M.; Wu, Y.; Nie, H.; Li, D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Świrk, K.; Rønning, M.; Motak, M.; Beaunier, P.; Da Costa, P.; Grzybek, T. Ce-and Y-modified double-layered hydroxides as catalysts for dry reforming of methane: On the effect of yttrium promotion. Catalysts 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar] [CrossRef]
- Dębek, R.; Zubek, K.; Motak, M.; Da Costa, P.; Grzybek, T. Effect of nickel incorporation into hydrotalcite-based catalyst systems for dry reforming of methane. Res. Chem. Intermed. 2015, 41, 9485–9495. [Google Scholar] [CrossRef]
- Izquierdo-Colorado, A.; Dębek, R.; Da Costa, P.; Gálvez, M.E. Excess-methane dry and oxidative reforming on Ni-containing hydrotalcite-derived catalysts for biogas upgrading into synthesis gas. Int. J. Hydrog. Energy 2018, 43, 11981–11989. [Google Scholar] [CrossRef]
- Kolebuk, I.; Samojeden, B. The Preparation and Proporties of Mg-and Ni-Modified Cenospheres; AGH University of Science and Technology: Kraków, Poland, 2018. [Google Scholar]




| Ni | SBET | H2 Consumption for the Calcined Samples | Basicity for the Calcined Samples after Reduction * | Basicity after DRM * | Nickel Crystallite Size for the Reduced Samples ** | Nickel Crystallite Size for the Spent Catalysts ** | Carbon Deposi-tion † | |
|---|---|---|---|---|---|---|---|---|
| wt % | m2/g | µmolH2/gcat | µmolCO2/gcat | µmolCO2/gcat | nm | nm | % | |
| Mg10 | ||||||||
| 10 | 1 | 53.4 | 35.0 | 29.0 | 20 | 23 | 0.5 | |
| 20 | 1 | 201.5 | 30.0 | 44.7 | 40 | 41 | 0.6 | |
| 30 | 2 | 455.5 | 27.1 | 28.1 | 36 | 39 | 0.9 | |
| Mg20 | ||||||||
| 10 | 1 | 82.86 | 29.7 | 19.6 | 23 | 24 | - | |
| 20 | 2 | 344.8 | 22.3 | 41.5 | 44 | 31 | - | |
| 30 | 4 | 947.5 | 18.3 | 21.5 | 43 | 41 | - | |
| Mg30 | ||||||||
| 10 | 2 | 233.7 | 19.9 | 36.0 | 32 | 34 | - | |
| 20 | 4 | 386.2 | 48.0 | 49.3 | 39 | 41 | 1.1 | |
| 30 | 4 | 612.3 | 26.0 | 35.9 | 36 | 37 | - |
| Catalyst | Ni Loading | Reaction Conditions | Conversion * | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | CH4/CO2 | GHSV (h−1) | TOS (h) | CH4 (%) | CO2 (%) | H2/CO | |||
| HTNi | 20 | 750 | 1/1 | 20,000 | 1 | 85 | 82 | 1.1 | [44] |
| HT-25Ni | 19.57 | 750 | 1/1 | 20,000 | 0.5 | 97 | 90 | 1.2 | [43] |
| HT | 20 | 750 | 1/1 | 20,000 | 0.5 | 82.5 | 86.5 | 0.93 | [41] |
| Mg20Ni20 | 20 | 750 | 1/1 | 20,000 | 0.5 | 97.9 | 93.9 | 0.98 | This work |
| Mg30Ni20 | 10 | 750 | 1/1 | 20,000 | 0.5 | 95.7 | 93.7 | 0.97 | This work |
| Mg30Ni20 | 20 | 750 | 1/1 | 20,000 | 0.5 | 96.7 | 93.8 | 0.91 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samojeden, B.; Kamienowska, M.; Izquierdo Colorado, A.; Galvez, M.E.; Kolebuk, I.; Motak, M.; Da Costa, P. Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures. Catalysts 2019, 9, 1066. https://doi.org/10.3390/catal9121066
Samojeden B, Kamienowska M, Izquierdo Colorado A, Galvez ME, Kolebuk I, Motak M, Da Costa P. Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures. Catalysts. 2019; 9(12):1066. https://doi.org/10.3390/catal9121066
Chicago/Turabian StyleSamojeden, Bogdan, Marta Kamienowska, Armando Izquierdo Colorado, Maria Elena Galvez, Ilona Kolebuk, Monika Motak, and Patrick Da Costa. 2019. "Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures" Catalysts 9, no. 12: 1066. https://doi.org/10.3390/catal9121066